International Journal of Anesthetics and Anesthesiology
Pre-Clinical Pharmacokinetics of Sufentanil-2-Hydroxypropyl-Β-Cyclodextrin Inclusion Complex
Silvana Aparecida Calafatti1, Marcelo de Macedo1, Juliana Zampoli Boava Papini1, Edvaldo Coelho1, Cíntia Maria Saia Cereda2, José Pedrazzoli Júnior1, Eneida de Paula2, Daniele Ribeiro de Araújo3 and Giovana Radomille Tofoli4*
1São Francisco University, Brazil
2Department of Biochemistry, Institute of Biology, State University of Campinas-UNICAMP, Brazil
3Human and Natural Sciences Center, Federal University of ABC, Brazil
4Institute and Research Center São Leopoldo Mandic, Brazil
*Corresponding author: Dr. Giovana R. Tofoli, Institute and Research Center São Leopoldo Mandic, Rua José Rocha Junqueira 13, 13045-75, Campinas, São Paulo, Brazil, Tel: +55 19 3211 3600, E-mail: giovana.tofoli@slmandic.edu.br
Int J Anesthetic Anesthesiol, IJAA-3-049, (Volume 3, Issue 3), Research Article; ISSN: 2377-4630
Received: April 12, 2016 | Accepted: July 15, 2016 | Published: July 18, 2016
Citation: Calafatti SA, de Macedo M, Papini JZB, Coelho E, Cereda CMS, et al. (2016) Pre-Clinical Pharmacokinetics of Sufentanil-2-Hydroxypropyl-Β-Cyclodextrin Inclusion Complex. Int J Anesthetic Anesthesiol 3:049. 10.23937/2377-4630/3/3/1049
Copyright: © 2016 Calafatti SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
This study evaluated the pre-clinical pharmacokinetics induced by sufentanil-2-hydroxypropyl-β-cyclodextrin inclusion complex (SUFHP-β-CD) in comparison with its aqueous formulation (SUF) after intramuscular injection in rabbits and pigs. New Zealand White rabbits and Landrace pigs were divided in two groups (n = 5/6) and treated by intramuscular route with SUF or SUFHP-β-CD complex (10 μg.kg-1). Blood samples were collected by a heparinized cannula pre dose (0 min) and at 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420 and 480 minutes after the injection of formulations in rabbits and pigs. Sufentanil plasma levels were determined using liquid chromatography-tandem mass spectrometry. Data were submitted to statistical (two-tailed unpaired t-test, p < 0.05) analysis. In both species, intramuscular injections of SUFHP-β-CD induced lower plasma concentrations than SUF at almost all periods of time (p < 0.05) and promoted smaller values for the maximum plasma concentration (Cmax) and the areas under the curve (AUC0-480) with SUFHP-β-CD (p < 0.05) when compared to SUF. Volume of distribution (Vd) was higher with SUFHP-β-CD (p < 0.05) in pigs and rabbits. These results showed that this cyclodextrin -based drug-delivery system of sufentanil was effective to reduce the absorption of the drug.
Keywords
Mass spectrometry, Absorption, Pharmacokinetics, Opioids, Drug delivery
Introduction
Opioids are used for the postoperative period, cancer pain and for moderate to severe chronic noncancer pain [1]. Sufentanil (SUF) is a highly lipophilic opioid that presents rapid and highly effective pain relief [2,3], but it presents short duration of action. Due to this short duration of action, SUF is currently used as an intravenous anesthetic agent and analgesic adjuvant for surgery and labor [4]. SUF produces adverse effects such as bradycardia, respiratory depression or excessive sedation [5]. Even though these adverse effects may occur, sufentanil produces less respiratory depressive effects relative to is analgesic effects which justify its use [4].
Development of new medicines is expensive and time consuming so that approaches to improve safety efficacy ratio of "old" drugs have been attempted, such as the use of drug delivery systems [6]. The use of these systems can prolong the effect of the drug or allow the achievement of equivalent effects with lower drug doses. The pharmacokinetics of the drug associated with carriers, such as cyclodextrins, usually shows more constant and lower plasma concentrations when compared to the free drug, suggesting delayed drug transfer to the bloodstream [7].
Previous papers reported that the inclusion complex of sufentanil in 10% hydroxypropyl-beta-cyclodextrin (HP-β-CD) increased the effectiveness of sufentanil after epidural and intrathecal administration in rats. After complexation sufentanil is available for a longer period at the spinal level, and, therefore less free sufentanil is available for redistribution, producing less systemic side-effects [8]. In another study, the complexation of sufentanil prolonged the spinal analgesic action and decreased the supraspinal actions of intrathecal drug, due to reduced diffusion into the vascular compartment [9].
A study of our group described a sufentanil-2-HP-β-CD inclusion complex that prolonged analgesic effect after intramuscular administration in rats and promoted slow release of SUF after in vitro release kinetics tests [10]. Although in vitro release tests are convenient, the results obtained may not correspond to the in vivo situation. Thus, the objective of this study was to evaluate the pre-clinical pharmacokinetic profile of this new intramuscular formulation of SUF complexed with HP-β-CD. Despite of the many therapeutics options, pain is still poorly controlled and might impair everyday activities and quality of life. Our experiment intended to evaluate a new tool in pain control that maybe useful in the future.
Material and Methods
Preparation of solid inclusion complex
Sufentanil citrate was a gift from Cristália Ind. Farm. Ltda. and HP-β-CD were purchased from Roquette Serv. Tech. Lab. The inclusion complex used in this study was identical to that described previously and presented the same in vitro characteristics [10]. The complex was obtained by mixing equimolar amounts of HP-β-CD and sufentanil (SUF) in deionized water, at room temperature (25 ± 1°C) for 24 h. After reaching equilibrium, the solution was freeze-dried in a Freezone® 4.5L freeze-dry system (Labconco, USA) and stored at -20°C for further use. Two milligrams of the solid inclusion complex (SUFHP-β-CD) were dissolved in 10 mL of HEPES solution (pH 7.40), SUF concentration in the final solution was 56.2 μg.mL-1.
Animals and pharmacokinetic study
The experimental protocol was approved by the Institutional Committee for Ethics in Animal Research of São Francisco University (protocol #001.12.09). This randomized blind study was conducted in two independent phases. During Phase I, twelve New Zealand White rabbits (2.50-3.00 kg) were divided into two groups (n = 6) and in Phase II ten Landrace pigs (25.00-30.00 kg) were divided into two groups (n = 5). The animals were treated by intramuscular route with SUF or SUFHP-β-CD complex (10 μg.kg-1). The dose used in this study was determinate in pilot study.
In our study design we decided to use two animal species since absorption, distribution and metabolic profile might be diverse in different species. We used the same place of injection and the same technique in both animal species. The place of the injection was the gluteal muscles of pigs and the needle used was a 18 G × 1 1/2 in. (BD®). Rabbits also received the injection in gluteal mass and the needle was a 25 G × 1 in. (BD®). The needle was inserted straight to ensure penetration into the muscle and not in the subcutaneous tissue.
During Phase I and II, an intravascular catheter was inserted in the ear vein of the animals and blood samples (1 mL) were collected via a heparinized cannula pre dose (0 min) and at 15, 30, 45, 60, 90, 120, 180, 240, 300, 360, 420 and 480 minutes after the injection of formulations. These intervals were defined during the pilot study to provide ten samples between the base line (0 min) and approximately 4 times the t1/2 (half-life time) of SUF (59.2 ± 22.0 min) [11]. Immediately after each blood collection plasma was separated and stored at -70°C until analysis.
LC-MS/MS assay
Sufentanil plasma levels were determined using a Waters® HPLC system (2795) coupled to a Micromass Quattro Premier XE triple stage quadrupole mass spectrometer equipped with an API electrospray source. All separations were carried out on a Phenomenex C18 (100 mm x 4.6 mm id, 5 μm particle size). The mobile phase was 70% acetonitrile and 30% water with 0.2% formic acid (pH = 3.5). The total run time was 2.5 minutes. The full-scan single-mass spectrum and the daughter ion-mass spectrum for sufentanil and lamivudine (internal standard - IS) were (m/z) 387.10 > 238.10 and 229.90 > 111.90, respectively. The data were integrated using the MassLynx 4.1 (Waters®) software. Quality controls samples (QC- 0.15; 48.00 and 96.00 ng.mL-1) were used to validate the method and were prepared by mixing drug-free plasma with appropriate volumes of working solutions.
The frozen plasma samples (200 μL) were thawed at room temperature and 50 μL of internal standard (IS) (lamivudine 3 μg/mL) were added. The samples were vortexed for two minutes and 1000 μL of hexane/ethyl acetate (1:1; V/V) were added. The samples were vortexed for five minutes and centrifuged at 1200×g, for 5 min at -4°C. The organic liquid layer was dried under nitrogen flow. After solvent evaporation, samples were reconstituted in 200 μL mobile phase and 0.15 mL were transferred to LC-MS/MS system vials, for further injection (5 μL).
Precision and accuracy of the analytical method were controlled by calculating the intra-batch and inter-batch variation at three concentrations of QC in five replicates (n = 5). Three calibration curves were plotted as the peak area ratio versus sufentanil concentration in the range of 0.05-120.00 ng.mL-1. The limit of quantification (LQ) was defined as the lowest concentration at which precision and accuracy were within 20% of the true value.
Pharmacokinetic assessment and statistical analysis
The concentration-time data were analyzed by the noncompartmental approach. The pharmacokinetic parameters were calculated using WinNonlin software (WinNonlin version 5.3, Pharsight Corporation, CA, US). Data were submitted to statistical analysis with unpaired t-test (α = 0.05).
Results and Discussion
The analysis of SUF was highly selective with the absence of interfering compounds and ion suppression at the retention times for SUF and IS. The calibration curves for SUF showed a good response over the range of 0.05-120.00 ng.mL-1. The assay was linear and coefficients (r2) were greater than 0.99 for all curves. Intra and inter- batch accuracy of QC plasma samples ranged from 97.66 to 108.95% and precision ranged from 2.23 to 9.49%. The LQ for SUF was 0.05 ng.mL-1. The results indicate that the method is reliable and reproducible within its analytical range.
After the intramuscular administration of the formulations in rabbits, SUFHP-β-CD induced lower plasma concentrations than SUF at all periods of time (p < 0.05), except at 360 min. In pigs, differences in plasma concentrations were observed at almost all periods of time (p < 0.05), except at 420 and 480 min. In our study aqueous formulation (SUF) did achieve higher plasma concentrations at almost all the sampling time, but this is not an advantage of the aqueous solution. On the contrary, opioids side effects are related to peak plasma concentrations and drug delivery systems (such as cyclodextrins) could reduce these occurrences [12,13]. All rabbits and pigs presented SUF in the systemic circulation after 15 minutes of the administration of the two formulations.
Previous in vitro evaluation of release kinetics showed that SUFHP-β-CD presents typical characteristics of reduced drug release: indeed total release of SUF was observed at 200 minutes of dialysis, when at the same time just 70% was released from SUFHP-β-CD (constant release values of 7.05 ± 0.52 min-1/2 and 5.61 ± 0.39 min-1/2 for SUFHP-β-CD and SUF, respectively) [10].The results obtained in vivo are in agreement with those. The plasma concentrations of SUFHP-β-CD after intramuscular administration were more constant and lower when compared to the free drug in almost all periods of time (Figure 1). This reduced absorption to plasma serves as a good indication of the slow-release profile of drugs delivered locally by cyclodextrins [7].
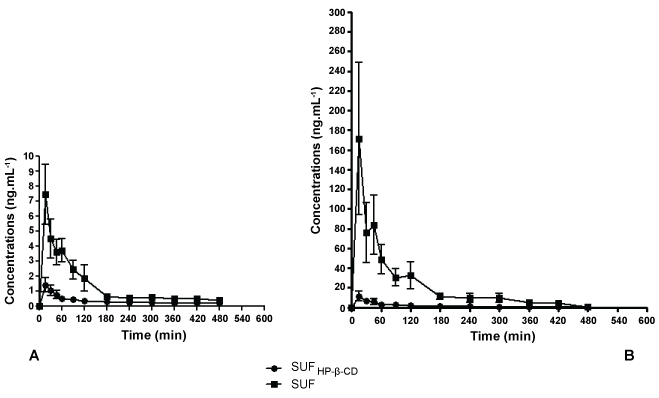
.
Figure 1: Graph of mean plasma concentration versus time after the injection of SUF and SUFHP-β-CD complex in rabbits (A) and pigs (B). Values are expressed as mean ± SEM. A: differences were observed at all periods of time (p < 0.05), except at 360 min; B: differences were observed at all periods of time (p < 0.05), except at 420 and 480 min.
View Figure 1
The intramuscular administration of SUFHP-β-CD and SUF in pigs and rabbits produced huge differences in the pharmacokinetic parameters related to absorption and distribution. In pigs the maximum plasma concentration (Cmax) values were approximately 24 times bigger in SUF group than in SUFHP-β-CD. Correspondingly, the areas under the curves (AUC0-480 and AUC0-∞) values were 16 and 12 times bigger in SUF group, respectively. Finally, volume of distribution (Vd) values in SUFHP-β-CD group were 19 times bigger when compared to SUF group. In rabbits the values of Cmax, AUC0-480 and AUC0-∞ were around 5; 4 and 2.5 times bigger for SUF, respectively. Vd was about 7 times bigger for SUFHP-β-CD (Table 1). These alterations in the pharmacokinetic parameters, especially in the absorption of the drug, could be explained by the fact that complexation with cyclodextrins might change the drug permeation across biologic membranes or its distribution [14,15]. Despite of the absence in differences in Tmax values, our formulation did produce reduced absorption and maintain constant drug concentration during the evaluated time interval. Extended or sustained release formulations are developed to maintain constant or prolonged concentrations of drugs. An ideal opioid extended release formulation would provide consistent pain control and minimization of adverse events associated with peak drug levels. Distinct technologies of formulations for opioids might produce varied release and pharmacokinetic profiles. The pharmacokinetic profile might present no lag time in drug absorption and exhibited a plasma concentration versus time profile with a sharp initial slope similar to immediate-release formulations followed by a sustained release phase [13], as our formulation behavior. Our group proposed a new formulation of sufentanil complexed with cyclodextrins designed for intramuscular use. We designed this study with two animal species to collect fundamental pre-clinical data in order to allow clinical evaluation in the future. Pigs and rabbits are good options to perform pharmacokinetic studies, especially because these animals present a higher volume of blood and easy ways to collect it when compared to rats. Also we decided to use "large experimental animal models" to observe extensive whole-body pharmacokinetics in a context comparable to patient physiology [16]. We observed that this new formulation was able to reduce the release and the absorption of SUF. Thus, the use of HP-β-CD may be an interesting strategy to diminish the liberation of SUF after intramuscular administration.
![]()
Table 1: Pharmacokinetic parameters after intramuscular injection of SUF and SUFHP-β-CD complex in rabbits and pigs (10 μg.kg-1). Data expressed as mean (± SD) (n = 5-6/group).
View Table 1
Acknowledgments
The authors thank (Cristália Produtos Quim. Farm. Ltda (SP, Brazil) for the donation of sufentanil and FAPESP (# 2009/17715-7) for the financial support. The authors also thank Mr. Edvaldo C. Coelho for his contribution in the pharmacokinetics analysis.
Financial Support
FAPESP (# 2009/17715-7).
References
-
Stanos SP, Bruckenthal P, Barkin RL (2012) Strategies to reduce the tampering and subsequent abuse of long-acting opioids: potential risks and benefits of formulations with physical or pharmacologic deterrents to tampering. Mayo Clin Proc 87: 683-694.
-
Aubrun F, Mazoit JX, Riou B (2012) Postoperative intravenous morphine titration. Br J Anaesth 108: 193-201.
-
Maciejewski D (2012) Sufentanil in anaesthesiology and intensive therapy. Anaesthesiol Intensive Ther 44: 35-41.
-
Minkowitz HS, Singla NK, Evashenk MA, Hwang SS, Chiang YK, et al. (2013) Pharmacokinetics of sublingual sufentanil tablets and efficacy and safety in the management of postoperative pain. Reg Anesth Pain Med 38: 131-139.
-
Vora KS, Shah VR, Patel B, Parikh GP, Butala BP (2012) Postoperative analgesia with epidural opioids after cesarean section: Comparison of sufentanil, morphine and sufentanil-morphine combination. J Anaesthesiol Clin Pharmacol 28: 491-495.
-
Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, et al. (2012) Drug delivery systems: An updated review. Int J Pharm Investig 2: 2-11.
-
de Paula E, Cereda CM, Fraceto LF, de Araújo DR, Franz-Montan M, et al. (2012) Micro and nanosystems for delivering local anesthetics. Expert Opin Drug Deliv 9: 1505-1524.
-
Meert TF, Mesens J, Verheyen P, Noorduin H (1992) Hydroxypropyl-beta-cyclodextrin can modulate the activity of spinally administered sufentanil. Eur J Anaesthesiol 9: 399-409.
-
Jang J, Yaksh TL, Hill HF (1992) Use of 2-hydroxypropyl-beta-cyclodextrin as an intrathecal drug vehicle with opioids. J Pharmacol Exp Ther 261: 592-600.
-
Volobuef C, Moraes CM, Nunes LA, Cereda CM, Yokaichiya F, et al. (2012) Sufentanil-2-hydroxypropyl-β-cyclodextrin inclusion complex for pain treatment: physicochemical, cytotoxicity, and pharmacological evaluation. J Pharm Sci 101: 3698-3707.
-
Bernards CM, Shen DD, Sterling ES, Adkins JE, Risler L, et al. (2003) Epidural, cerebrospinal fluid, and plasma pharmacokinetics of epidural opioids (part 1): differences among opioids. Anesthesiology 99: 455-465.
-
Lauretti GR, Mattos AL, Lima IC (1997) Tramadol and beta-cyclodextrin piroxicam: effective multimodal balanced analgesia for the intra- and postoperative period. Reg Anesth 22: 243-248.
-
Kizilbash A, Ngô-Minh CT (2014) Review of extended-release formulations of Tramadol for the management of chronic non-cancer pain: focus on marketed formulations. J Pain Res 7: 149-161.
-
Stella VJ, He Q (2008) Cyclodextrins. Toxicol Pathol 36: 30-42.
-
Kurkov SV, Loftsson T (2013) Cyclodextrins. Int J Pharm 453: 167-180.
-
Mather LE, Copeland SE, Ladd LA (2005) Acute toxicity of local anesthetics: underlying pharmacokinetic and pharmacodynamic concepts. Reg Anesth Pain Med 30: 553-566.





