International Journal of Cancer and Clinical Research
Dosimetric Study on the Consequences of Replacing the mMLC Collimator Used for Intracranial SRS by an Integrated MLC-160
Tania Santos1*, Tiago Ventura2, Miguel Capela2 and Maria do Carmo Lopes2
1Physics Department, University of Coimbra, Portugal
2Medical Physics Department, Instituto Portugues de Oncologia de Coimbra Francisco Gentil, Portugal
*Corresponding author:
Tania Santos, Physics Department, University of Coimbra, Coimbra, Portugal, Rua Larga 3004-516 Coimbra, Portugal, Tel: +351 239410600, E-mail: taniafssantos@gmail.com
Int J Cancer Clin Res, IJCCR-3-064, (Volume 3, Issue 4), Original Article; ISSN: 2378-3419
Received: March 03, 2016 | Accepted: August 15, 2016 | Published: August 17, 2016
Citation: Santos T, Ventura T, Capela M, Lopes MC (2016) Dosimetric Study on the Consequences of Replacing the mMLC Collimator Used for Intracranial SRS by an Integrated MLC-160. Int J Cancer Clin Res 3:064. 10.23937/2378-3419/3/4/1064
Copyright: © 2016 Santos T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: At IPOCFG, stereotactic radiosurgery is performed using an add-on micro-multileaf collimator (3 mm minimum leaf width), m3, with full advanced integration in a linear accelerator equipped with a standard 82 leaf multileaf collimator with 1 cm leaf width. This work aimed to evaluate if it would be possible to dispense the use of the m3 if the standard MLC was replaced by a MLC-160 with 5 mm leaf width at isocenter.
Methods and materials: 67 metastases and 23 meningiomas were chosen to form the basis of this retrospective planning study as representing the whole variety of treated cases. For each clinical case two treatment plans were generated: one using the m3 and the other using the MLC-160. The dosimetric differences from both plans were quantified in terms of target coverage, dose conformity and organs at risk sparing. The Wilcoxon signed rank test was performed to evaluate statistical differences.
Results: The characteristics associated with the MLC-160 system (larger leaf width and static conformal arcs) contributed to a poorer dose conformity, mainly for irregular shaped or small lesions and for lesions at close proximity to OARs. Statistically significant differences were found in terms of the minimum dose for lesions ≤ 0.5 cm3 and irregular lesions. As a consequence, almost 35% of the considered clinical plans would not be clinically acceptable if the intended replacement of the collimation system would take place.
Conclusion: The mMLC associated with the dynamic arc technique yields dosimetric benefits in radiosurgery. The treatment of lesions with complex shapes or at close proximity to OARs do benefit from the used system.
Keywords
Micro-multileaf collimator, Leaf width, Stereotactic radiosurgery, Plan quality
Introduction
Stereotactic Radiosurgery (SRS) is a treatment technique characterized by the administration of high doses of radiation in a single fraction with high accuracy to a small and well-defined intracranial target, while limiting the dose to surrounding normal tissues [1].
In the early development of linear accelerator-based (LINAC) systems, circular collimators of different diameters coupled to the LINAC were used and the SRS treatment was performed with multiple convergent and non coplanar arcs [2,3]. Since then, LINAC-based SRS has become highly sophisticated. The introduction of the multileaf collimator (MLC) has incorporated new features to radiotherapy and also the SRS process, enabling the generation of irregular field shapes allowing improvements in conformity and homogeneity of the dose distribution [1].
The impact of the narrower leaf widths on radiotherapy treatment planning has been studied by many authors [4-11]. Kubo, et al. [7] analysed the impact of the MLC leaf width on stereotactic radiosurgery and 3D conformal radiotherapy treatment plans, using 1.7, 3 and 10 mm leaf width MLC systems. They showed that for radiosurgery treated lesions, the 1.7 and 3 mm leaf width allowed to comply the RTOG treatment planning guidelines for SRS, in most cases.
Burmeister, et al. [9], in a study with patients treated using intensity-modulated radiotherapy demonstrated that the replacement of a 10 mm by a 5 mm MLC system would be clinically benefic in cases involving very small or irregular target volumes. More recently, Tanyi, et al. [10] performed a dosimetric comparison between MLC systems with 2.5 mm and 5 mm leaf width for the SRS treatment of 68 intracranial lesions and demonstrated small dosimetric benefits of the 2.5 mm leaf width MLC system over the 5 mm leaf width system.
The present study aimed at justifying a management decision on the replacement of the standard 82-leaf Optifocus MLC collimator (1 cm leaf width at isocenter) installed in the Siemens ONCOR Avant-Garde linear accelerator. We would like to evaluate if the replacement of the 82-leaf MLC by a 160 MLC with 5 mm leaf width at isocenter would enable to dispense the use of the add-on BrainLab mMLC (m3) presently used for SRS without significantly jeopardizing the quality of the treatment plans. This evaluation would include quantifying the amount of the eventual losses in terms of plan quality and assessing the characteristics of the lesions that would mostly be affected by the eventual replacement.
Methods
At IPOCFG SRS is performed since February 2008 using the micro-multileaf collimator mMLC of BrainLab (3 mm minimum leaf width) -m3, with full advanced integration in a Siemens ONCOR Avant-Garde linear accelerator using the 6 MV photon mode. The treatment technique consists in 6-7 dynamic conformal arcs rotating about a single isocenter using the m3 to conform to the target volume every 10° incidence.
Patients selection
For the proposed retrospective planning study, 67 metastatic brain lesions and 23 meningiomas treated with SRS between November 2008 and December 2014 have been selected from the overall pool of treated cases as representing its variety. The clinical cases have thus been categorised into four groups: (I) brain metastases with volume ≤ 0.5 cm3 (n = 36), (II) brain metastases with volume > 0.5 cm3 (n = 16), (III) brain metastases located in close proximity to organs at risk (n = 15) and (IV) meningiomas (n = 23) (Table 1).
![]()
Table 1: Summary of group's characteristics.
View Table 1
The groups were created to represent the different clinical situations in our SRS experience. Groups I and II were intended to compare the volume effect, group III for critical organ sparing and group IV was chosen for shape effect assessment. As meningioma cases may appear in any brain location, including at close proximity to critical structures, some cases in group IV were also compared for critical organ sparing.
Collimators
The m3 has 26 pairs of leaves: from centre to periphery 14 pairs with 3 mm width, six pairs with 4.5 mm and six pairs with 5.5 mm width. The maximum possible field size is 10 × 10 cm2 at isocenter. It is a tertiary or additional collimator which must be attached to the linac head every time a stereotactic treatment is to be delivered. The irradiation technique is based on dynamic conformal arcs using in almost all cases a single isocenter for each lesion. During irradiation, the gantry is rotating while the leaves of the mMLC move according to the beam’s eye view (each leaf of the m3 moves linearly at 10° intervals interpolating from the initial position to the next calculated position).
The Siemens 160 Multileaf Collimator has 160 leaves -80 on each bank with a leaf width of 5 mm at isocenter over the full maximum field size of 40 × 40 cm2. It is integrated on the linac head replacing the X-jaws. The irradiation technique is based on static conformal arcs as the ONCOR Avant-Garde linac does not include the option for dynamic irradiation mode. The radiation field is adjusted to the largest shape of the target lesion in just one incidence per arc. During irradiation, the gantry is rotating and the MLC shape remains static.
Machine profiles comparison
The current study is not just a simple comparison of different MLC leaf widths. It also includes the assessment of two dose delivery systems associated to each MLC.
The micro-multileaf collimator of BrainLab is the collimator system used at our institution for SRS treatments. The corresponding basic dosimetry was locally performed during the commissioning process and the dosimetric database was stored in the Physics Administration module (PAM) of the treatment planning system (iPlan, BrainLab). For the present study, BrainLab provided a full dosimetric database of an external linear accelerator of the same model (Oncor) equipped with a Siemens MLC-160 which was stored as a new Machine Profile in PAM.
Before starting the retrospective planning study, a dosimetric comparison between the basic dosimetry databases corresponding to the two delivery systems was performed to evaluate if the characteristics of the radiation field were comparable and to validate the external machine dosimetry. For the m3 the local nominal linac output for a 10 × 10 cm2 field size with source surface distance of 100 cm and at a depth of 5 cm was 0.867 Gy/100 MU. For the external MLC-160 system the nominal linac output was defined for a 10 × 10 cm2 with source surface distance of 100 cm and at a depth of 10 cm as 0.677 Gy/100 MU. A renormalization of the nominal output for the MLC-160 to a depth of 5 cm was made and the obtained value of 0.872 Gy/100 MU differs from that of m3 in just 0.6%. The depth dose profiles were also compared for the common field sizes: 3 × 3 cm2, 6 × 6 cm2, 8 × 8 cm2 and 10 × 10 cm2. The obtained depth dose profiles coincided within less than 1%, with larger differences for smaller fields, mainly at the build-up region.
Based on the previous comparisons, we have assumed that the two dosimetric machines could be used for the proposed planning study.
Treatment planning
Treatment planning is locally conducted using iPlan treatment planning system, version 4.5 from BrainLAB. Contrast enhanced magnetic resonance (MR) images of each patient are co-registered with planning computed tomography (CT) images acquired in a big-bore CT simulator (Siemens Sensation Open). A head ring frame fixation system and the corresponding CT-localizer are used to enable the required stereotactic transformation performed at the planning workstation. The neuroradiologist approves the image fusion and delineates the gross tumour volume (GTV) and the relevant organs at risk (OARs) on the MR images. Subsequently, the planning target volume (PTV) is defined as the GTV plus an isotropic margin of 1mm incorporating the clinical target volume (CTV) and accounting for setup errors and other possible isocenter localizing uncertainties.
For the present study, reference plans were created for m3. The plans used for treatment have not been used to prevent planner skills dependence. All clinical cases were re-planned with both MLCs using exactly the same treatment planning parameters, such as the isocenter location, the number of non-coplanar conformal arcs, the MLC margin, the gantry start and stop angles, the collimator and table rotation angles for each arc, and the prescription dose.
Treatment plans were computed using a pencil beam algorithm for 6 MV photon beam energy. A dose resolution of 1 mm was set. The adaptive grid option was used for the calculation of the dose matrix. In this option the dose grid size is automatically adapted to the volume of small structures guaranteeing at least 10 voxels for dose computation in all directions.
Plan quality evaluation parameters
Target coverage (TC) [12], conformation number (CN) [13], conformity index (CI) [12], Conformity/Gradient Index (CGI) [14] and the volume of normal tissue receiving at least 100% of the prescription dose (VNormPI) were used to compare the treatment plans. The definition of each index is summarized below. PTV dose-volume histogram (DVH) parameters including minimum dose and maximum dose were computed and recorded. DVHs for the critical structures were compared in terms of the dose received by 0.1 cm3 of brainstem () as well as the volume of critical organ that receives more than its tolerance dose and more than the prescribed dose.
Target coverage: The dose coverage of the target volume was evaluated using the coverage index defined by Lomax and Scheib in 2003 [12] as:
Where VT,pi is the volume of target that receives at least the prescription dose and VT the PTV volume. Ideally TC would be 100%. The general acceptance criteria is TC ≥ 95%.
Dose conformity: To avoid possible false high scores, dose conformity was quantified using CN and CI. CI, proposed by Lomax and Scheib in 2003 [12] is defined as the ratio between the volume of target receiving at least the prescription dose and the volume enclosed by the prescription isodose, Vpi:
The CN index was proposed Van’t Riet, et al. in 1997 [13] and combines two ratios: the proportion of the target covered by the prescription dose (equivalent to TC) and the proportion of the prescription isodose volume that covers the target (equivalent to CI):
CN and CI should be greater than 0.6 for the plan to be considered conformal.
Conformity/Gradient index: Conformity/Gradient Index (CGI) proposed by Wagner, et al. in 2003[14] was defined as the average of two terms, the conformity score (CGIc), and the gradient score (CGIg) given as follows:
where REff, pi is the effective radius of the prescription isodose volume and REff,50%pi is the effective radius of the 50% isodose volume.
The effective radius of a volume is the radius of a sphere of equal volume, and is calculated as:
The final CGI index value is given by:
The CGI increases with plan desirability or quality, such that an ideal plan with CGIc = 100 and CGIg = 100 would have a CGI score of 100. As either the conformity or the gradient worsens, CGI will decrease.
Improvement ratio: A ratio to evaluate the improvement in each dose-volume index and the minimum and maximum dose to PTV between the two rival plans (a plan obtained with the m3 vs. a plan obtained with the MLC-160) was defined as:
For the dose-volume indices and the minimum dose to PTV a positive improvement ratio means that MLC-160 would performed better than m3 in terms of the corresponding index. When the maximum dose to PTV is evaluated, a positive improvement ratio means that the MLC-160 would contribute to a more heterogeneous dose distribution, what means a lower performance of MLC-160 vs. m3.
Statistical analysis
Statistical analysis was carried out using MATLAB version R2014a. The Wilcoxon signed rank test for paired samples was performed to assess differences between plans, with a p-value < 0.05 defining statistical significance.
Results
PTV coverage, dose conformity and conformity/gradient index
The dosimetric indices and their improvement ratios according to the MLC system (MLC-160 vs. m3) for the four groups of clinical cases are summarized in table 2. The Wilcoxon signed rank tests the null hypothesis which is that the median difference between pairs of observations is zero. Therefore, the p-value column in the table exhibits the probability of rejecting the null hypothesis (if p < 0.05 the indices are statistically different and the improvement ratio is real, either positive or negative).
![]()
Table 2: Average quality indices and corresponding improvement ratios (± 1σ) of MLC-160 versus m3 systems.
View Table 2
Target coverage: An identical PTV coverage was achieved using the MLC-160 and the m3 for groups I, II and III (p > 0.05). For group IV despite that the differences were statistically significant, the improvement ratio is negative and modest being on average less than 1% (-0.91 ± 1.19%). This means that the MLC-160 would cover the targets just a little bit less, to 97% on average, instead of the 98% level achieved by the m3, for this type of irregular lesions as meningiomas.
Conformity: CI and CN indices indicate unequivocal statistically significant differences between m3 and MLC-160 systems for all groups in terms of dose conformity. Differences were higher for lesions with smaller volume (≤ 0.5 cm3, group I) or irregular shaped lesions (group IV). When using the MLC-160 system, dose conformity deteriorated, on average, by around 9% for group I, 4% for group II, 6-7% for group III and 11-12% for group IV.
Conformity/Gradient index: Regarding CGI improvement ratios were shown to be negative and of around 3% for groups I, III and IV and just of around 1% for group II, indicating the lower performance of the MLC-160 system.
Minimum and maximum target doses
Minimum and maximum doses to the PTV are shown in table 3. No statistically significant differences were found between m3 and MLC-160 systems for all groups, in terms of maximum dose to the PTV. Differences in terms of minimum dose were statistically significant for groups I and IV, with negative improvement ratios, which means that for lesions close to OARs and/or irregularly shaped, the MLC-160 would lead to higher underdosage of the PTV.
![]()
Table 3: Average values of minimum and maximum doses (± 1σ) to the PTV and corresponding improvement ratios for MLC-160 vs. m3 systems.
View Table 3
Dose to critical structures
For group III and group IV brainstem sparing was studied. Group III includes 15 clinical cases of brain metastases located near the brainstem. Group IV includes 23 clinical cases of meningiomas. Meningiomas appeared in any brain location, including at close proximity to brainstem, optical nerves or chiasm. For this group, in 14 cases the lesion was located near the brainstem. In just two cases the chiasm and optic nerves were the considered OARs. Therefore, the brainstem was taken as the more relevant OAR to be reported for groups III and IV.
The dose received by 0.1 cm3 of brainstem (D0.1cm3) the volume of brainstem that received at least 12 Gy(V ≥ 12Gy) and the volume of brainstem that received at least the prescription isodose (V ≥ pi) are summarized in table 4. For clinical cases in which the dose received by the brainstem was greater than its tolerance dose (12 Gy), a plan was considered clinically unacceptable when D0.1cm3 and/or V ≥ 12 Gy and/or V ≥ pi were greater than in the plan approved for treatment (for equivalent conformity and target coverage).
![]()
Table 4: Brainstem sparing average values (± 1σ) for both collimator systems.
View Table 4
Figure (1a) and figure (1b) displays D0.1cm3 received by brainstem for all clinical cases included in group III and for the clinical cases of group IV where the PTV is located at close proximity to the brainstem (n = 14), using the m3 and the MLC-160 systems, respectively.
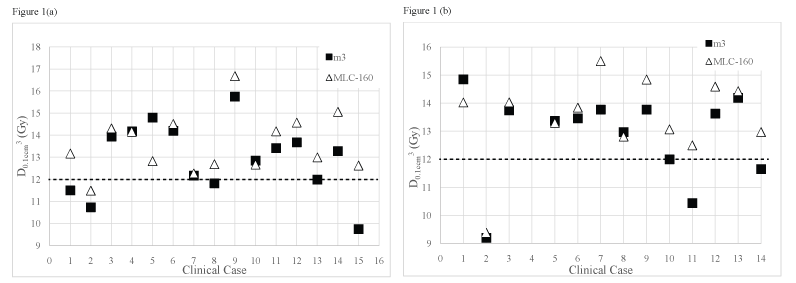
.
Figure 1: Dose received by 0.1 cm3 of brainstem for (a) Group III (metastases close to the brainstem); (b) Group IV (meningiomas located close to the brainstem) obtained using the m3 and the MLC-160 systems. The dashed line represents the brainstem tolerance dose, 12 Gy.
View Figure 1
A quantitative evidence of improved brainstem sparing for the m3 over the MLC-160 system is shown. The MLC-160 contributed on average for an increase in the dose received by 0.1 cm3 of brainstem of around 1 Gy. When the MLC-160 was used, also the volume of brainstem that received at least its tolerance dose (V ≥ 12 Gy) is on average, 1.83 times greater than the volume that received the same dose when the m3 was used for group IV. Also for group III this difference is statistically significant but smaller (overdosed brainstem volume 1.27 times greater with MLC-160). The volume of brainstem that received at least the prescribed isodose is on average larger for the MLC-160 system in both groups. Although being almost three times greater in group IV, the absolute volume of brainstem receiving at least the prescription dose is reduced, not surpassing 0.23 cm3, on average.
Dose to normal tissue
The absolute volume of normal brain tissue irradiated to at least the prescribed dose for all lesions is summarized in table 5 (second and third columns). The fourth and fifth columns are respectively the difference in volume for the two MLC systems and this volume in percentage to the PTV volume.
![]()
Table 5: Average absolute volume (± 1σ) of normal tissue irradiated to at least 100% of the prescribed dose, VNormPI.
View Table 5
For all lesion groups there was a statistically significant difference (p < 0.05).
According to the types of target, the differences for both MLC systems in terms of VNorm100% relatively to the VPTV ranged, on average, from around 6% in group II to around 20% in group IV. It is important to evaluate both absolute and relative volumes as, for instance, in group I, the volumetric difference between the two MLC systems is just 0.04 cm3 but this small volume represents already more than 13% of the average volume of the metastases in this group. Therefore for group I, III and IV the MLC-160 system would lead to higher irradiation of the normal tissue.
Discussion
Studies on the impact of linear accelerator MLC leaf width on stereotactic radiosurgery and radiotherapy plans have demonstrated that thinner MCL leaf widths yield dosimetric benefits in cases involving complex target/organ-at-risk geometry [4-11].
Four groups of clinical cases were created to evaluate the eventual quality loss in treatment plans if the add-on mMLC of BrainLab (3 mm minimum leaf width -m3) presently used for SRS was replaced by an integrated MLC with 5.0 mm leaf width, the Siemens 160 Multileaf Collimator (MLC-160). Plan comparisons included the use of dose-volume data, volumes of normal adjacent tissue and irradiated critical structures, as well as indices to evaluate target coverage and dose conformity.
The plan quality differences mainly result from the different leaf widths and from the impossibility of using dynamic arc technique with the MLC-160. The same planner has done all the plans for the present study and has tried her best to achieve the best possible plan for each clinical case with the two MLC systems, without any time pressure. The average difference between the planning time for the worse cases for the two MLC systems was estimated to be around 40 minutes, in favour of m3.
The results demonstrated that identical PTV coverage would be achieved using either the m3 or the MLC-160 systems. Regarding CGIg, dose falloff outside the target was not significantly different for m3 and MLC-160, so the observed statistically significant difference in terms of CGI index was due to a poorer conformity provided by the MLC-160, which is in accordance with what CN and CI indices values revealed.
Concerning the maximum dose received by the PTV, the differences between the two MLC systems were not statistically significant so the dose homogeneity would not change with the introduction of the MLC-160 in the clinical practice. Significant statistical differences were found in terms of the minimum dose to the PTV for groups I (lesions ≤ 0.5 cm3) and IV (meningiomas). However, the improvement ratio for small metastases (≤ 0.5 cm3) was just -0.69% ± 1.10% which may not be clinically significant as for both MLCs the achieved minimum doses were above 95% of the prescribed dose. The minimum dose to the PTV in groups III and IV were always lower than 95% of the prescribed dose which means that close to OARs with lower tolerance doses than the prescribed dose, a compromise is usually established between proper PTV coverage and critical organ sparing trying to reduce toxicity. In these cases, the statistically significant difference obtained for group IV between the minimum dose to the PTV with the m3 and the MLC-160 systems may have significant clinical impact. We can preview that the use of the MLC-160 would probably lead to more significant underdosage of irregular lesions close to organs at risk.
Dose conformity was consistently better for the m3 plans that for MLC-160 plans. The effect of target volume was compared for groups I and II. As expected, the dose conformity was higher for larger lesions. Although the conformity of the MLC-160 plans was systematically poorer than that of the m3 plans, both CN and CI indices were, on average, greater than 0.6 which means that the MLC-160 treatment plans would be clinically acceptable as conformal.
Group III included brain metastases located at close proximity or within the brainstem. Therefore organ at risk sparing was a limiting factor that had to be taken into account during planning. Good conformity, with average and median conformity indices above 0.6, could be achieved with both MLC systems as was shown in table 2. However the conformity index would assume poorer values in 6-7% if the m3 was replaced by the MLC-160.
The fourth group was formed by meningiomas which are typically irregular lesions. This group had the poorest conformity. With the MLC-160 system the plans could not achieve acceptable conformity, on average. The defined improvement ration assumed the highest negative average values (around -12%), meaning that the plan conformity would suffer important decrease with the MLC replacement.
We believe that the higher differences between the two MLC systems in terms of dose conformity for group IV relatively to group III were due to the meningioma irregular shapes. For irregular shaped lesions, the static conformal arc irradiation would represent the highest limitation in the scenario of MLC replacement.
The volume of normal tissue irradiated to at least the prescription dose was consistently greater for plans using the MLC-160 over the m3 for all groups but in higher amount for groups I, III and IV. Table 4 shows that the MLC-160 system would also contribute for a higher organ at risk affectation. For clinical cases of meningioma or metastases close to organs at risk, therapeutic dose and lesions location could compromise the brainstem tolerance dose, as D0.1cm3 values showed.
Conclusion
If the tertiary 3 mm micro-multileaf collimator of BrainLab presently used for stereotactic radiosurgery was replaced on the Siemens Avant-Garde linear accelerator by an integrated Siemens 160 MLC with 5 mm leaf width, organs at risk sparing and dose conformity of lesions located at close proximity to organs at risk and highly irregular shaped lesions would be the main losses in terms of plan quality. Another important result from the study and still linked with the previously referred ones was that the minimum dose to the PTV would significantly be reduced for the larger width leaf MLC system, what would contribute to poorer clinical outcome or higher toxicity as a trade-off.
The main reason for the observed results was not just the larger leaf width of MLC-160 but the fact that this system integration in the local linear accelerator would have the limitation of not enabling dynamic conformal arcs for the SRS technique.
As a consequence, treatment planning using the MLC-160 has been a cumbersome and time consuming task for all considered groups of lesions, mainly for irregular shaped lesions and lesions located near eloquent areas. The planning time has easily been doubled when compared to the planning time using the m3 collimator which is not an irrelevant issue when real treatment cases are to be considered in SRS where the treatment planning must be done on the same day as the treatment, unless some pre-planning strategy is adopted [15].
Considering all clinical studied cases, 5.6% (2/36) of the cases included in group I, 33.3% (5/15) in group III and 34.8% (8/23) in group IV would not be clinically acceptable if the intended replacement of the collimation system would take place. Therefore the eventual MLC replacement would not represent a beneficial option considering the local SRS treatment experience.
PACS Numbers
87.53.Ly, 87.55D, 87.56.nk
References
-
Siyong K, Palta J (2008) The Physics of Stereotactic Radiosurgery. In: Chin SL, Regine FW, Principles and Practice of Stereotactic Radiosurgery. New York, Springer, 33-49.
-
Betti OO, Derechinsky VE (1984) Hyperselected encephalic irradiation with linear accelerator. Acta Neurochir 33: 385-390.
-
Colombo F, Benedetti A, Pozza F, Zanardo A, Avanzo RC, et al. (1985) Stereotactic radiosurgery utilizing a linear accelerator. Appl Neurophysiol 48: 133-145.
-
Monk JE, Perks JR, Doughty D, Plowman PN (2003) Comparison of a micro-multileaf collimator with a 5-mm-leaf-width collimator for intracranial stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 57: 1443-1449.
-
Chern SS, Leavitt DD, Jensen RL, Shrieve DC (2006) Is smaller better? Comparison of 3-mm and 5-mm leaf size for stereotactic radiosurgery: A dosimetric study. Int J RadiatOncol Biol Phys 66: 76-81.
-
Jin J-Y, Yin F-F, Ryu S, Ajlouni M, Kim JH (2005) Dosimetric study using different leaf-width MLCs for treatment planning of dynamic conformal arcs and intensity modulated radiosurgery. Med Phys 32: 405-411.
-
Kubo HD, Wilder RB, Pappas CTE (1999) Impact of collimator leaf width on stereotactic radiosurgery and 3D conformal radiotherapy treatment plans. Int J Radiat Oncol Biol Phys 44: 937-945.
-
Wu QJ, Wang Z, Kirkpatrick JP, Zheng Chang, Jeffrey J Meyer, et al. (2009) Impact of collimator leaf width and treatment technique on stereotactic radiosurgery and radiotherapy plans for intra- and extracranial lesions. Radiat Oncol 4:3.
-
Burmeister J, McDermott PN, Bossenberger T, Ben-Josef E, Levin K et al. ( 2004) Effect of MLC leaf width on the planning and delivery of SMLC IMRT using the CORVUS inverse treatment planning system. Med Phys 31: 3187-3193.
-
Tanyi JA, Kato CN, Chen Y, Chen Z, Fuss M (2011) Impact of the high-definition multileaf collimator on linear accelerator-based intracranial stereotacticradiosurgery. Br J Radiol 84: 629-638.
-
Marrazzo L, Zani M, Pallotta S, Greto D, Scoccianti S, et al. (2014) Comparison of stereotactic plans for brain tumors with two different multileaf collimating systems. J Appl Clin Med Phys 15: 27-37.
-
Lomax NJ, Scheib SG (2003) Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys 55: 1409-1419.
-
Van't Riet A, Mak ACA, Moerland MA, Elders LH, Van Der Zee W (1997) A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: Application to the prostate. Int J Radiat Oncol Biol Phys 37: 731-736.
-
Wagner TH, Bova FJ, Friedman WA, Buatti JM, Bouchet LG, et al. (2003) A simple and reliable index for scoring rival stereotactic radiosurgery plans. Int J Radiat Oncol Biol Phys 57: 1141-1149.
-
Ahn K-H, Ozturk N, Smith B, Slavin KV., Koshy M, et al. (2016) A preplanning method for stereotactic radiosurgery to improve treatment workflow. J Appl Clin Med Phys 17: 171-179.





