Journal of Rheumatic Diseases and Treatment
Salivary Glands Ultrasonography in Rheumatoid Arthritis Patients. Its Role in Early Detection of Secondary Sjogren's Syndrome
Amal Mohamad El-Barbary1*, Marwa Ahmed Aboelhawa1, Abeer Abdelmenem Shahba2, Noha Mohamed Shafik3, Hala Elsayed Hamouda3 and Hossam Abdelhafiz Zaytoun4
1Department of Rheumatology and Rehabilitation, Faculty of Medicine, Tanta University, Egypt
2Department of Internal Medicine, Faculty of Medicine, Tanta University, Egypt
3Department of Medical Biochemistry, Faculty of Medicine, Tanta University, Egypt
4Department of Radiology, Faculty of Medicine, Tanta University, Egypt
*Corresponding author: Amal Mohamad El-barbary, MD, Assistant Professor, Department of Rheumatology and Rehabilitation, Faculty of Medicine, Tanta University, Egypt, Tel: +201001264626, E-mail: ml_barbary@yahoo.com
J Rheum Dis Treat, JRDT-2-041, (Volume 2, Issue 4), Research Article; ISSN: 2469-5726
Received: July 26, 2016 | Accepted: September 24, 2016 | Published: October 01, 2016
Citation: El-Barbary AM, Aboelhawa MA, Shahba AA, Shafik NM, Hamouda HE, et al. (2016) Salivary Glands Ultrasonography in Rheumatoid Arthritis Patients. Its Role in Early Detection of Secondary Sjogren's Syndrome. J Rheum Dis Treat 2:041. 10.23937/2469-5726/1510041
Copyright: © 2016 El-Barbary AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: To investigate the use of ultrasonic to assess the salivary glands in RA patients presenting with sicca symptoms, and its correlations with clinical, laboratory and minor salivary gland biopsy.
Methods: One hundred and fifty RA patients classified into two equal subgroups according to the presence of sicca symptoms, in addition to seventy five healthy controls. All patients were subjected to self-reported questionnaire about sicca symptoms, DAS 28, Unstimulated whole salivary flow rate (UWSFR), Schirmer's test, ESR, CRP, RF, Anti- CCP, Anti-Ro, Anti-La antibodies, serum and salivary CXC ligand 13 (CXCL13), B cell activating factor (BAFF), ultrasonography for major salivary gland and biopsy of minor salivary gland.
Results: 93.3% of RA patients with sicca symptoms and 9.3% of RA patients without sicca symptoms revealed significant pathological changes in salivary ultrasonography. The sensitivity of ultrasonography in early detection of secondary Sjogren's syndrome in RA patients was 93.3% and the specificity was 90.67%. There were significant association ultrasonography changes with serum and salivary levels of CXCL13 and BAFF (p < 0.001). Salivary glands ultrasonography is negatively correlated with UWSFR and positively correlated with salivary levels of CXCL13 and BAFF (p < 0.001- p < 0.05) and salivary gland biopsy (p < 0.001).
Conclusions: Submandibular ultrasonography is a promising practical technique that can be used for detection of pathological changes in secondary Sjogren's syndrome in RA.
Keywords
Salivary glands ultrasonography, Rheumatoid arthritis, Sjogren's syndrome
Introduction
Secondary Sjogren's syndrome (SS) was occurring in approximately 30% of RA patients [1] where involvement of exocrine lacrimal and salivary glands occurs. The decrease of salivary and lacrimal functions in RA is assumed to be related to the lymphocytic infiltrate present in the affected glands. Secondary SS is manifested by dry eyes (Keratoconjuntivitis sicca) and dry mouth (xerostomia) [2]. The diagnosis of SS faces some difficulties; it was found that the average time between occurrence of the first symptoms and diagnosis of SS was 7.1 years [3].
Diagnosis of SS has traditionally depended on signs, symptoms, serologic test, and invasive procedures such as minor salivary gland biopsy. Work from several independent groups has shown common, consistent, and characteristic findings on parotid and minor salivary gland ultrasound among patients with SS. Salivary ultrasonography is a new method that holds the possibility of noninvasively providing important diagnostic information about major salivary glands in SS without radiation or surgery [4].
It was found that B cells have been implicated in the pathogenesis of SS. The chemokine CXC ligand 13 protein (CXCL13) is a CXC subtype member of the chemokine superfamily. Chemokines have been shown to orchestrate migration and preferential sequestration of B and T cells in inflammatory lesions. Moreover, the B cell-activating factor belonging to the TNF family (BAFF) was shown to increase the chemotactic response of both naïve and memory B cells to CXCL13 [5].
The aim of this work is to evaluate the use of ultrasonic techniques to assess the parotid and submandibular glands in RA patients presenting with sicca symptoms and correlates its findings with clinical characteristics and serum and salivary levels of CXCL13 and BAFF.
Materials and Methods
Inclusion criteria
The study was carried out on one hundred and fifty patients with rheumatoid arthritis diagnosed according to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 criteria for diagnosing of RA [6]. They were selected from the Rheumatology and Rehabilitation Department, Tanta University Hospitals. The patients were classified into two equal subgroups according to the presence or absent of oral and ocular sicca symptoms (dry eyes daily for more than 3 months, sensation of sand or gravel, use of tear substitutes more than three times a day, dry mouth daily for more than 3 months, drink liquids to swallow dry food, and recurrently or persistently swollen salivary glands).
Seventy-five healthy volunteers matched in age and sex participated in the study as controls. Informed consents were taken from each patient and control. The study was approved by Local Research Ethics Committee of Faculty of Medicine, Tanta University.
Exclusion criteria
We excluded patients with ophthalmologic complications such as scleritis; episcleritis; scleromalacia; those with prior eye surgery; contact lenses users; those who were taking xerogenic drugs including neuroleptics, antidepressants, antihypertensive, parasympatholytic drugs, and so on; those with diabetes mellitus, hepatitis C; or HIV infection; or prior irradiation of the neck.
All Patients were Subjected to the Following Assessment
Clinical assessment
1. Self-reported questionnaire: The questionnaire comprised questions about oral and ocular sicca symptoms based on European-American classification criteria for SS [7].
2. DAS 28: DAS 28 was measured for RA disease activity that takes into account the number of swollen and tender joints, a measurement of general health by the patients, and values of sedimentation rate [8].
3. Unstimulated whole salivary flow rate (UWSFR) test: UWSFR was determined in the morning. Patients had not eaten, smoked, swallowed liquids, or rinsed their mouths for at least 1 hour before the test. Flow rates were expressed as ml/minute. Saliva production ≤ 1.5 ml/15 minutes was considered abnormal [9]. Xerostomia was defined as one or more oral sicca symptoms in combination with pathological UWSFR [10,11].
4. Schirmer's test: The standardized tear test strips were placed between the medial and lateral parts of the lower eyelid without preceding use of anesthetic eye drops. After 5 minutes the strips were removed, and the length of the wetted area of the strip was measured. The test considered positive if the length of the wetted area was ≤ 5 mm at one or both eyes [12]. Keratoconjuntivitis sicca was defined as one or more ocular sicca symptoms in combination with pathological Schirmer's test [10,11].
Laboratory assessment
Sampling: Seven ml of venous blood were withdrawn from patients and control subjects under complete aseptic precautions, 1.6 ml blood was transferred into a vacutainer tube containing 0.4 ml sodium citrate for determination of ESR, 1 ml was placed in EDTA containing vacutainer for complete blood count, and the rest of the blood was delivered in a plain glass tube, allowed to clot at room temperature, and centrifuged at 2000 rpm for 10 minutes; the serum was separated. Rheumatoid factor (RF) and CRP were determined immediately, and aliquots of the rest of the serum and salivary samples were stored at -70°C till the time of assay of other laboratory tests. Saliva was centrifuged at 1500 rpm for 10 minutes before being analyzed.
Both patients and control groups were subjected to the following laboratory investigations:
1) Complete blood count was done using Advia 60 cell counter (Bayer).
2) Erythrocyte sedimentation rate (ESR) mm/first hour was determined by Westergren method.
3) C-reactive protein (CRP) in serum was determined by using CRP-latex slide agglutination test provided by (SPINREAT, S.A.U, and Spain).
4) Rheumatoid factor (RF) was determined by nephelometry method (Behring, Marburg, Germany).
5) Serum anti-cyclic citrullinated peptides (anti-CCP2) measured by ELISAs (Quanta Lite CCP version 3.1 for IgG/IgA from Inova Diagnostics, San Diego, CA).
6) Serum Anti-Ro (Anti-SS-A) and Serum Anti-La (Anti-SS-B) antibodies were determined by quantitative sandwich enzyme linked immunoassay technique (ELISA), provided by ORGENTEC Diagnostika GmbH, Germany, according to the manufacturer's instructions: normal value: < 5 U/ml, borderline: 15-25 U/ml and elevated: > 25 U/ml [13].
7) Assessment of CXCL13 in serum and saliva: CXCL13 levels were determined by quantitative sandwich enzyme linked immunoassay technique (ELISA), provided by RayBiotech, Inc [14].
8) Assessment of BAFF in serum and saliva: BAFF levels were assessed using a quantitative sandwich enzyme immunoassay technique (Quantikine® Human BAFF Immunoassay, R&D Systems, Minneapolis, Minnesota, USA). Results are presented in pg/ml [15].
Radiological assessment: Ultrasonography for parotid gland and submandibular gland: sonographic examinations were performed using a B-K Medical (Mileparken 34, Denmark). Real-time scanner with center frequency of 6 to 12 MHz linear transducer. Bilateral parotid glands of each patient were scanned in two planes, parallel and perpendicular to the retromandibular plane. Bilateral submandibular glands of each patient were scanned in two planes, parallel and perpendicular to the submandibular plane. Ultrasonic examination was done by two radiologists blinded to the clinical diagnosis.
The parenchymal structure of the glands was categorized into five stages: stage 0: normal, stage 1: mild parenchymal inhomogeneity (PIH) (hypoechoic areas < 2 mm), stage 2: evident PIH (hypoechoic areas of 2-6 mm), stage 3: gross PIH (hypoechoic areas > 6 mm) and stage 4: adipose degeneration of the gland (adipose tissue echogenicity and parenchymal atrophy) [16,17].
Biopsy of minor salivary gland: Biopsy of minor salivary gland from the inner side of the lower lip was done in all included patients and sections were stained by hematoxylin-eosin and considered positive (focus score of ≥ 1) when a focus of 50 lymphocytes/4 mm 2 was found. Salivary gland biopsy was read by a blinded pathologist. Secondary Sjogren's syndrome is diagnosed in RA patients according to revised international classification criteria for Sjogren's syndrome, by the American-European Consensus Group (AECG) [7].
Statistical analysis
All data were analyzed using SPSS software (version11; SPSS Inc., Chicago, IL, USA). Baseline characteristics are presented as mean ± standard deviation or as median (interquartile range) for continuous variables, and as frequency (percentage) for discrete variables. Comparisons between groups and association between characteristics of patients and ultrasonography (US) grading were conducted using analysis of variance (ANOVA) and Fisher LSD test. Cohen's kappa test was used for the analysis of inter-observer variation. Correlation between variables was examined using Pearson's correlation coefficient. p value < 0.05 was considered statistically significant. Sensitivity and specificity were calculated for sonography and biopsy of salivary gland examinations.
Results
The study included 150 patients with RA. The patients were classified into two equal subgroups according to the presence or absent of oral and ocular sicca symptoms. All RA patients were under disease-modifying antirheumatic drugs (DMARDs) either as monotherapy or in combination.
The main demographic, clinical, and serological characteristics of RA patients and controls are summarized in table 1. There was a significant difference between the two groups of RA patients, according to Schirmer's test, and unstimulated whole salivary flow rate (UWSFR) (p < 0.001).
![]()
Table 1: Demographic, clinical and serological parameters in RA patients and controls.
View Table 1
There was no significant difference between the two groups of RA patients, according to DAS 28 laboratory parameters, including ESR, CRP, RF and anti-CCP2 levels. Moreover, there was a significant difference between the two groups of RA patients and controls according to anti-Ro and anti-La levels. In the RA group with sicca symptoms only five patients were positive to anti-Ro (6.6%), and four patients were positive to anti-La (5.3%). Whereas none of the RA groups without sicca symptoms were positive to these autoantibodies.
Regarding serum and salivary CXCL13 and BAFF levels, CXCL13 and BAFF levels were significantly increased in RA patients compared to controls with insignificant differences between two groups of RA patients regarding serum levels of CXCL13. However, serum BAFF, salivary CXCL13, and salivary BAFF were significantly increased in the RA group with sicca symptoms compared to the RA group without sicca symptoms (p < 0.001). Ocular manifestations presented more than oral manifestations in RA patients with sicca symptoms. Moreover, 4% of RA patients without sicca symptoms revealed pathological Schirmer's and UWSFR tests table 2 and table 3.
![]()
Table 2: The percentage of associated sicca symptoms in RA patients presented with sicca symptoms.
View Table 2
![]()
Table 3: Signs of 2ry Sjogren's syndrome RA patients.
View Table 3
In this study, 93.3% of RA patients with sicca symptoms and 12% of RA patients without sicca symptoms revealed positive labial salivary gland biopsy. However, 93.3% of RA patients with sicca symptoms and 9.3% of RA patients without symptoms revealed pathological changes in salivary ultrasonography. 80% of RA patients with sicca symptoms had ultrasonic abnormalities in submandibular glands, whereas 33.3% of patients had ultrasonic abnormalities in parotid glands.
The sensitivity of the labial salivary gland biopsy in early detection of secondary S in RA patients was 93.3%, and the specificity was 88%. However, sensitivity of salivary gland ultrasonography was 93.3%, and the specificity was 90.67%.
Regarding the salivary gland ultrasonography grading, in the RA group with sicca symptoms, forty patients out of seventy (57.14%) had grade 3 ultrasonic changes (Figure 1), whereas twenty-seven out of seventy patients (38.5%) had grade 2 ultrasonic changes, and only three patients had grade 1 changes (4.2%). However, 9.3% of RA group without sicca symptoms revealed grade 2 ultrasonic changes. The kappa test for inter-observer variation was good (0.891). There was a significant association between salivary gland ultrasonography grading with serum and salivary levels of CXCL13 and BAFF in RA patients (Figure 2) (p < 0.001).
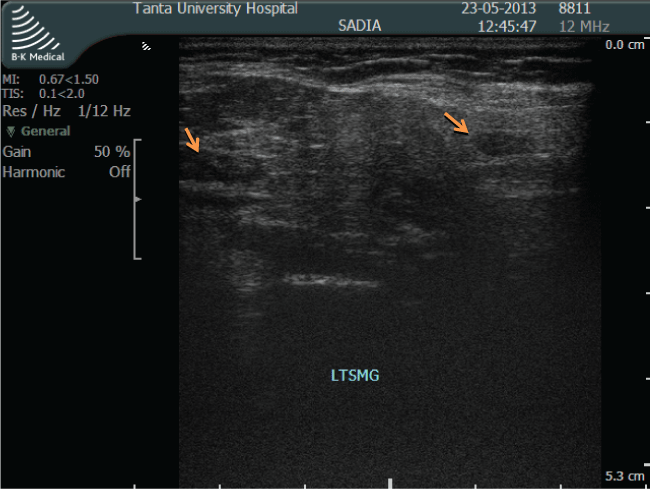
.
Figure 1: Ultrasound image of grade 3 abnormalities in submandibular gland, gross parenchymal inhomogenicity (hypoechoic areas > 6 mm).
View Figure 1
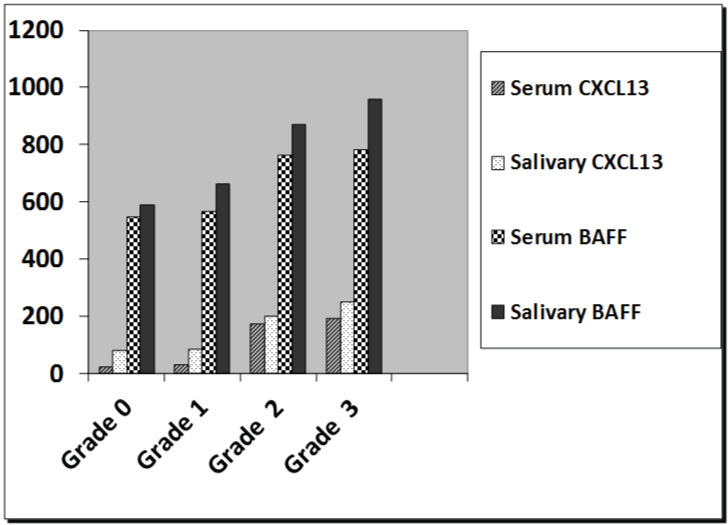
.
Figure 2: The relation between salivary glands ultrasonography grading with serum and salivary levels of CXCL13 and BAFF in RA patients.
View Figure 2
Regarding correlation, there was a negative correlation between serum and salivary levels of CXCL13 and BAFF with Schirmer's and UWSFR tests in RA patients with sicca symptoms (r = -0.330, p = 0.01; r = -0.638, p < 0.001).
Salivary gland ultrasonography grading was negatively correlated with UWSFR tests (r = -0.64, p < 0.001). However, Salivary gland ultrasonography grading is positively correlated with laboratory tests (salivary levels of CXCL13 and BAFF levels (r = 0.70, p < 0.001; r = 0.45, p < 0.05) and salivary gland biopsy (r = 0.64, p < 0.001).
Discussion
Although RA is known as a disease of the joints, it is important to recognize that it is a systemic disease often affecting extra-articular tissues throughout the body including the skin, blood vessels, heart, lungs, and muscles. The most common extra-articular manifestation of RA is SS, manifested by dry eyes (Keratoconjuntivitis sicca) and dry mouth (xerostomia) [18]. Fujita, et al. [19], Salliot, et al. [20], Tomiak, et al. [21], and Amador, et al. [22] reported that the coexistence of SS and RA was 5%, with 30% caused by an autoimmune epithelitis in lacrimal and salivary glands.
Regarding the RA patients who showed the sicca symptoms, it was found that the patients who suffered from ocular manifestations were greater than the patients who suffered from oral manifestations. Lemp [23] demonstrated that ocular manifestation was presented for more than 90% of the RA patients. Moreover, Wengkaew, et al. [11] documented that the prevalence of ocular and oral subjective symptoms in RA patients were 38% and 6%, respectively.
In the RA group with sicca symptoms, only five patients showed positive results for the anti-Ro (6.6%), and four patients showed positive results for anti-La (5.3%). These results agreed with Franceschini, et al. [24], Cavazzana, et al. [25], and Yoshimi, et al. [26], who found that anti-Ro antibodies were detected in 3 to 15% of patients with RA associated with extra-articular features. The low frequency of anti-Ro/La antibodies among RA patients confirmed that these antibodies are unlikely to be contributory in this group of patients.
In this study, CXCL13 and BAFF levels were significantly high in RA patients when compared to controls. Serum BAFF, salivary CXCL13, and salivary BAFF levels were significantly high in the RA group with sicca symptoms when compared to RA group without sicca symptoms. Levels of CXCL13 and BAFF were negatively correlated with Schirmer's and UWSFR tests. Moreover, the higher levels were associated with a higher degree of ultrasonic changes in salivary glands.
CXC ligand13 (CXCL13), which is also known as B cell-attracting chemokine1 or B lymphocyte chemoattractant, is a member of the CXC subtype of the chemokine superfamily. It is critical for secondary lymphoid tissue development and distribution of lymphocytes within microenvironments. The synergy between BAFF and CXCL13 might also favor the recruitment of pathological B cells and their sequestration in follicle-like structures during autoimmune diseases [27].
Kramer, et al. [28] found that CXCL13 increased with disease progression in salivary tissue and serum from SS models. Furthermore, 74% of the patients with primary SS had increased levels of serum and salivary CXCL13. Moreover, BAFF overexpression that has been observed in SS is associated with B-cell tolerance breakdown and autoantibody production. In the salivary glands, a minority of B-cell clusters represent ectopic germinal center cells, while the majority manifest features that were consistent with transitional type 2 (T2) and marginal-zone (MZ)-like B cells. Interestingly, both types of B-cell clusters, including autoreactive B cells and BAFF, is associated with MZ-like B cells in the salivary glands. Daridon, et al. [29] can identify the cells that produce BAFF in the salivary glands of patients with primary SS.
Kramer, et al. [30] concluded that salivary CXCL13 would enhance the diagnostic algorithm for sjogren syndrome significantly. In addition, CXCL13 measurements may have predictive value to develop serious oral disease manifestations.
Regarding radiological findings, pathological changes of salivary gland ultrasonography were detected in 93.3% of RA patients with sicca symptoms and 9.3% of RA patients without sicca symptoms. The sensitivity and specificity of salivary gland ultrasonography percentages were 93.3% and 90.67%, respectively. Moreover, salivary gland ultrasonography was significally correlated with salivary levels of CXCL13 and BAFF and salivary gland biopsy.
These results agreed with Tzioufas, et al. [31], who documented that ultrasonography was a noninvasive method that provided information about the changes to major salivary glands during inflammation. Also, a published study by Wernicke, et al. [32] documented that ultrasonography revealed the decreased level of echogenicity of submandibular glands in patients with SS but not in normal individuals, with a specificity of more than 90% and sensitivity close to 60%.
Milic, et al. [33] found that ultrasonographic abnormalities of salivary glands were detected in 107/115 (93.0%) of patients with primary SS, in 12/44 (27.3%) with secondary SS, in 25/50 (50.0%) with sicca symptoms, and in 4/36 (11.1%) asymptomatic controls. The sensitivity to specificity ratio was 91/83. Takagi, et al. [34] reported that the results from the submandibular ultrasound were significantly the same as the results from sialography for the diagnosis of SS. In addition, Salaffi, et al. [35] concluded that the findings of parotid and submandibular glands were equal.
From this study, we concluded that submandibular ultrasonography of RA was a promising technique that could be used as a practical noninvasive and sensitive technique for early detection of pathological changes for secondary SS in RA patients.
References
-
Manthorpe R, Frost-Larsen K, Isager H, Prause JU (1981) Sjögren's syndrome. A review with emphasis on immunological features. Allergy 36: 139-153.
-
Andonopoulos AP, Drosos AA, Skopouli FN, Acritidis NC, Moutsopoulos HM (1987) Secondary Sjögren's syndrome in rheumatoid arthritis. J Rheumatol 14: 1098-1103.
-
Segal B, Bowman SG, Jonsson R, Moutsopoulos HM, Alexander EL, et al. (2009) Primary Sjogren's syndrome: Health experiences and predictors of health quality among patients in the united states. Health Qual life Outcomes 7: 46.
-
Daniels TE (2012) Do we need new diagnostic criteria for Sjögren's syndrome? Presse Med 41: e441-449.
-
Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P (2007) Role of chemokines in endocrine autoimmune diseases. Endocr Rev 28: 492-520.
-
Aletaha D, Neogi T, Silman AJ, Felson DT, Wolfe F, et al. (2010) Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69: 1580-1588.
-
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, et al. (2002) Classification criteria for Sjogren's syndrome: A revised version of the European criteria proposed by the American- European Consensus Group. Ann Rheum Dis 61: 544-548.
-
Prevoo ML, Van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, et al. (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38: 44-48.
-
Navazesh M, Kumar SK; University of Southern California School of Dentistry (2008) Measuring salivary flow: Challenges and opportunities. J Am Dent Assoc 139: 35S-40S.
-
Gilboe IM, Kvien TK, Uhlig T, Husby G (2001) Sicca symptoms and secondary Sjögren's syndrome in systemic lupus erythematosus: Comparison with rheumatoid arthritis and correlation with disease variables. Ann Rheum Dis 60: 1103-1109.
-
Wengkaew S, Kasitanon N, Sivasomboon C, Wickainun R, Sukitawut W, et al. (2006) Sicca symptoms in Thai patients with rheumatoid arthritis, systemic lupus erythematosus and scleroderma: A comparison with age-matched controls and correlation with disease variables. Asian Pac J Alergy Immunol 24: 213-221.
-
Javadi MA, Feizi S (2011) Dry eye syndrome. J Ophthalmic Vis Res 6: 192-198.
-
Froelich CJ, Wallman J, Skosey JL, Teodorescu M (1990) Clinical value of an integrated ELISA system for the detection of 6 autoantibodies (ssDNA, dsDNA, Sm, RNP/Sm, SSA, and SSB). J Rheumatol 17: 192-200.
-
Carlsen Hs, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P (2004) Monocyte-like and mature macrophages produce CXCL13 (B cell- attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood 104: 3021-3027.
-
Liu Z, Davidson A (2011) BAFF and selection of autoreactive B cells. Trends Immunol 32: 388-394.
-
Niemela RK, Takalo R, Paakko E, Suramo I, Salo T, et al. (2004) Ultrasonography of salivary glands in primary Sjogren's syndrome. A comparison with magnetic resonance imaging and magnetic resonance sialography of parotid glands. Rheumatology 43: 875-879.
-
Jousse-Joulin S, Devauchelle-Pensec V, Morvan J, Guias B, Pennec Y, et al. (2007) Ultrasound assessment of salivary glands in patients with primary Sjögren's syndrome treated with rituximab: Quantitative and Doppler waveform analysis. Biologics 1: 311-319.
-
Rustin MH, Isenberg DA, Griffiths MH, Gilkes JJ (1988) Sjögren's syndrome and pleomorphic T-cell lymphoma presenting with skin involvement. J R Soc Med 81: 47-49.
-
Fujita M, Igarashi T, Kurai T, Sakane M, Yoshino S, et al. (2005) Correlation between dry eye and rheumatoid arthritis activity. Am J Ophthalmol 140: 808-813.
-
Salliot C, Mouthon L, Ardizzone M, Sibilia J, Guillevin L, et al. (2007) Sjogren's syndrome is associated with and not secondary to systemic sclerosis. Rheumatology (Oxford) 46: 321-326.
-
Tomiak C, Dorner T (2009) Diagnosis and therapy of Sjogren's syndrome in rheumatoid arthritis. Journal fur Mineralstoffwechsel 16: 24-31.
-
Amador-Patarroyo MJ, Arbelaez JG, Mantilla RD, Rodriguez-Rodriguez A, Cárdenas-Roldán J, et al. (2012) Sjögren's syndrome at the crossroad of polyautoimmunity. J Autoimmun 39: 199-205.
-
Lemp MA (2005) Dry eye (Keratoconjunctivitis Sicca), rheumatoid arthritis, and Sjögren's syndrome. Am J Ophthalmol 140: 898-899.
-
Franceschini F, Cavazzana I, Malacarne F, Airo P, Cattaneo R, et al. (2003) Anti-Ro/SSA antibodies in rheumatoid arthritis(RA). Arthritis Res Ther 5: 8.
-
Cavazzana I, Franceschini F, Quinzanini M, Manera C, Del Papa N, et al. (2006) Anti-Ro/SSA antibodies in rheumatoid arthritis: clinical and immunologic associations. Clin Exp Rheumatol 24: 59-64.
-
Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y (2012) Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol 2012: 606195.
-
Badr G, Borhis G, Lefevre EA, Chaoul N, Deshayes F, et al. (2008) BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood 111: 2744-2754.
-
Kramer JM, Rothstein TL (2012) Autoantibody levels correlate with CXCL13 in human Sjogren's syndrome. AADR Annual Meeting.
-
Daridon C, Devauchelle V, Hutin P, Le Berre R, Martins-Carvalho C, et al. (2007) Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum 56: 1134-1144.
-
Kramer JM, Klimatcheva E, Rothstein TL (2013) CXCL13 is elevated in Sjögren's syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol 94: 1079-1089.
-
Tzioufas AG, Moutsopoulos HM (2008) Ultrasonography of salivary glands: an evolving approach for the diagnosis of Sjögren's syndrome. Nat Clin Pract Rheumatol 4: 454-455.
-
Wernicke D, Hess H, Gromnica-Ihle E, Krause A, Schmidt WA (2008) Ultrasonography of salivary glands -- a highly specific imaging procedure for diagnosis of Sjögren's syndrome. J Rheumatol 35: 285-293
-
Milic VD, Petrovic RR, Boricic IV, Radunovic GL, Pejnovic NN, et al. (2010) Major salivary gland sonography in Sjögren's syndrome: diagnostic value of a novel ultrasonography score (0-12) for parenchymal inhomogeneity. Scand J Rheumatol 39: 160-166.
-
Takagi Y, Kimura Y, Nakamura H, Sasaki M, Eguchi K, et al. (2010) Salivary gland ultrasonography: can it be an alternative to sialography as an imaging modality for Sjogren's syndrome? Ann Rheum Dis 69: 1321-1324.
-
Salaffi F, Carotti M, Iagnocco A, Luccioli F, Ramonda R, et al. (2008) Ultrasonography of salivary glands in primary Sjogren's syndrome: a comparison with contrast sialography and scintigraphy. Rheumatology (Oxford) 47: 1244-1249.





