Journal of Rheumatic Diseases and Treatment
1National Institute of Rheumatic Diseases, Piešťany, Slovak Republic
2Department of Rheumatology and Rehabilitation, Thomayer Hospital, Prague 4, Czech Republic
*Corresponding author: Jozef Rovenský, National Institute of Rheumatic Diseases, Piešťany, Slovak Republic, E-mail: rovensky.jozef@nurch.sk
J Rheum Dis Treat, JRDT-2-043, (Volume 2, Issue 4), Review Article; ISSN: 2469-5726
Received: August 13, 2016 | Accepted: October 07, 2016 | Published: October 10, 2016
Citation: Rovenský J, Sedláčková M (2016) Relapsing Polychondritis. J Rheum Dis Treat 2:043. 10.23937/2469-5726/1510043
Copyright: © 2016 Rovenský J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Relapsing polychondritis (RP) is a rare immune-mediated disease that may affect multiple organs. It is characterised by recurrent episodes of inflammation of cartilaginous structures and other connective tissues, rich in glycosaminoglycan. Clinical symptoms concentrate in auricles, nose, larynx, upper airways, joints, heart, blood vessels, inner ear, cornea and sclera.
Diagnosis of the disease is based on the Minnesota diagnostic criteria of 1986 and RP has to be suspected when the inflammatory bouts involve at least two of the typical sites - auricular, nasal, laryngo-tracheal or one of the typical sites and two other - ocular, statoacoustic disturbances (hearing loss and/or vertigo) and arthritis.
The disease is treated with non-steroidal anti-inflammatory drugs or glucocorticoids in combination with other immunosuppressants. Currently, biological therapy is being researched for use in treatment of severe resistant forms. This therapy may play a role in treatment of severe resistant and relapsing forms.
Keywords
Relapsing polychondritis, Inflammatory damage to cartilage, RP primary and secondary forms, RP treatment with classical immunosuppressants and glucocorticoids, Biological therapy
Historical Background
The first to describe the disease was Jaksch-Wartenhorst from the Faculty of Medicine of the German University in Prague, in 1923 [1], who called it polychondropathy. Later the disease was referred to as systemic chondromalacia, panchondritis, chronic atrophic polychondritis and rheumatoid chondritis. The most fitting term for this disease was introduced by Pearson in 1960 who called it relapsing polychondritis (RP).
Epidemiology
A total of more than 860 cases of this rare disease were reported in the world literature. Its onset is most likely between the ages of 40 and 60, although it may occur also shortly after birth and in advanced age. Familial aggregation of the disease is unknown, and no correlation has been found between increased RP incidence and HLA Class I antigens.
The incidence of RP is estimated to be 3.5 cases per million population per year. Five-year survival has been recorded in 74% of patients; in the systemic vasculitis subgroup survival is similar to that of patients with polyarteritis (up to about five years in 45% of patients). The period of survival is reduced mainly due to infection and respiratory compromise.
Etiology and Pathogenesis
The most prominent RP manifestation is inflammation of cartilaginous structures resulting in their destruction and fibrosis. It is characterised by a dense inflammatory infiltrate, composed of neutrophil leukocytes, lymphocytes, macrophages and plasma cells. At the onset, the disease affects only the perichondral area, the inflammatory process gradually leads to loss of proteoglycans, destruction of the collagen matrix and ultimately to chondrocyte necrosis. The damaged cartilage is replaced by granulation and fibrous tissue.
The cause of the disease remains unknown but detailed studies have shown that immunological processes play an important role in the pathogenesis. Cartilage consists of collagen, proteoglycans and elastin that have many antigen determinants of normally sequestered cells of the immune system. Impaired integrity of the cartilaginous structure could be an important stimulus for the immunological response to these components that are contained in the cartilage of the respiratory system, the eye structures and the cardiovascular system. Specific antibodies against type II collagen, primarily IgG, were detected in the serum of 50% of patients with RP. Ebringer, et al. [2] found high titres of antibodies against type II collagen in the early phase of the disease that may be related to its activity. However, it should be noted that these antibodies are not RP-specific and were identified also in rheumatoid arthritis (RA); they are directed against various epitopes of the collagen molecule. A contributing factor to RP development may be also humoral immunity, as indicated by the findings of Arundell and Haserick [3] who described a case of RP development in a newborn of a mother with RP. The child was later cured. Deposits of immunoglobulins and complement were found in the chondrofibrous interface of the affected auricles, indicating involvement of immune complexes in the disease pathogenesis. Reduced complement concentration was detected in the middle ear fluid. These findings suggest that humoral immunity may participate in RP development, but a role of cell-mediated immunity in this respect should be considered as well, because cellular immunity is involved in immunity reactions together with proteoglycans and collagen.
Clinical Features
The subjective symptoms include auricular and nasal pain and tension, sometimes eye pain and arthralgia. Episodes of inflammation of the cartilage of one or both auricles and nose often develop suddenly and last for several days. Recurrent protracted inflammations destroy cartilage and produce various deformities, e.g., a saddle-nose deformity and red and swollen auricles tender to palpation (Figure 1). In the Slovak Republic, a report of the RP course was published by Tomík, et al. [4].
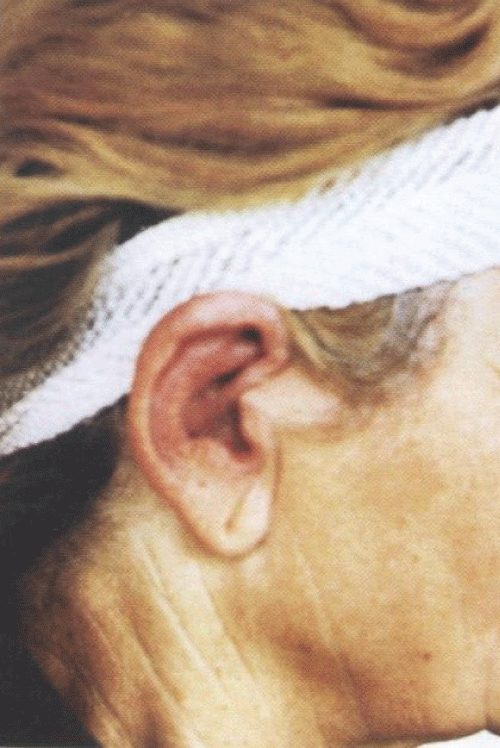
.
Figure 1: Red and swollen auricles tender to palpation in relapsing polychondritis.
View Figure 1
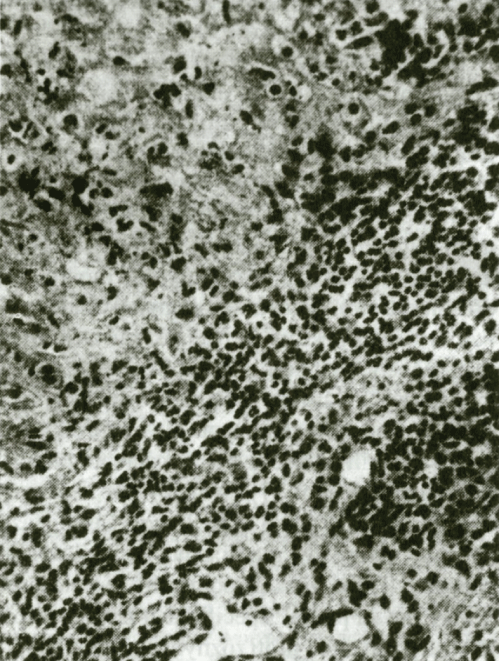
.
Figure 2: Progressive changes in cartilage of ear. (Hematoxylin and eosin staining, enlarged 250x).
View Figure 2
Arthropathy in relapsing polychondritis
RP quite often affects joints usually independently of other manifestations. The clinical features show episodic asymmetrical inflammation of large and small joints, including parasternal articulations and sacroiliac joints that lasts for several days or weeks. In general, it manifests itself as a non-deforming, non-erosive and seronegative (RF negative) polyarthritis. Radiographic examination of joints as a rule reveals joint space narrowing without erosion, which is given by the fact that the pathological process causes only loss of the hyaline cartilage. A typical histopathological finding shows a lighter space with multinucleated bodies around chondrocytes and unspecific proliferation of epithelial cells in the middle and deep layers of the synovial membrane. The clinical course as well as radiographic and histopathological findings serve as the basis for distinguishing pure RP polyarthritis from polyarthritis of the rheumatoid type (Figure 2, Figure 3 and Figure 4).
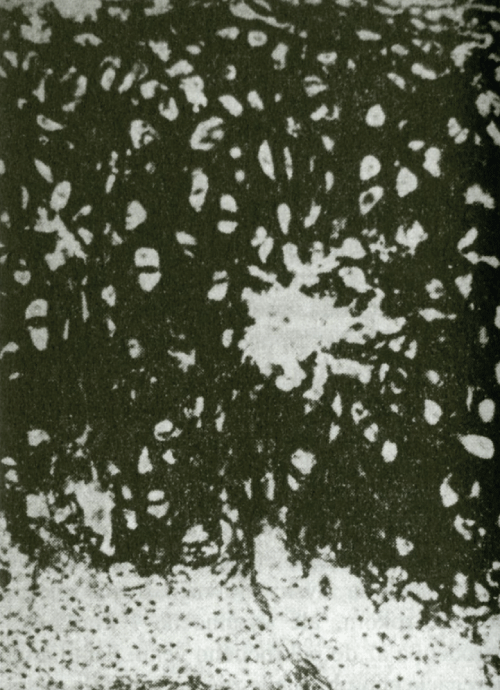
.
Figure 3: Homogenization and destruction of elastic fiber. (Stained by resorcin-fuchsin, enlarged 250x).
View Figure 3
Organ manifestations of relapsing polychondritis
In a half of the patients RP affects the respiratory system which may result in a breakdown of the architecture of bronchi and trachea and air collapse during the respiratory cycle. First, it usually involves larynx and the upper part of trachea together with the subglottic space. The process may progress and affect also the lower part of the trachea and main bronchi. Dominant clinical symptoms include dysphonia, cough, stridor and dyspnoea. During the active phase of the disease, there may occur increased tenderness over the thyroid cartilage and the trachea. Neilly, et al. [5] have found out that young patients with upper airways compromise developed as early as at the onset of the disease, are often resistant to the treatment and have a poor prognosis. Involvement of the respiratory system may be the only dominant symptom of the disease and may be mistaken for a banal chronic bronchitis. In such case it is beneficial to establish the diagnosis on the basis of flow-volume curve that will reveal an abrupt onset of acute obstruction of the upper airways (Figure 5).
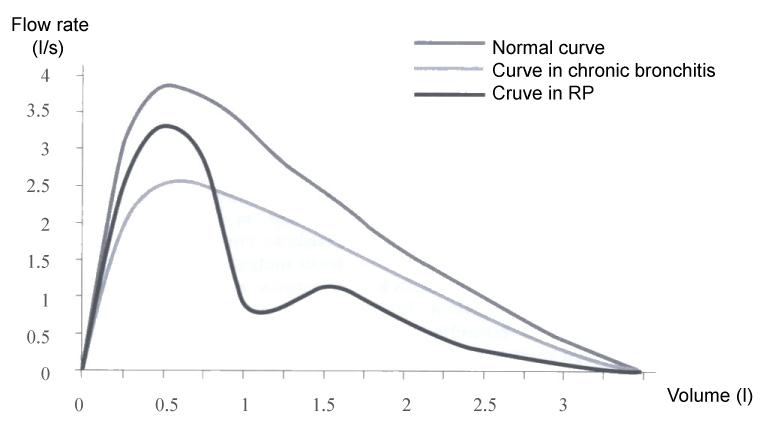
.
Figure 5: Expiratory flow curve showing peak flow rates at various lung volumes distal obstruction (asthma, chronic bronchitis) affects primarily the flow at low lung volumes, while proximal obstruction (trachea) the flow at high lung volumes.
View Figure 5
The cardiovascular system is involved in about 10% of patients, particularly in the form of thoracic and abdominal aortic aneurysms. Another disorder may be aortitis causing thinning of the media and aortic root dilatation with ruptures of the aortic valve. Systemic polyarteritis nodosa was identified in 9% of cases. The range of effects of inflammation on the blood vessels is quite broad. If it affects small blood vessels it has a form of cutaneous leukocytoclastic vasculitis, while in large blood vessels that of the Takayasu's arteritis. Inflammation may affect also the aortic and mitral valves and cause their functional insufficiency due to aortic root dilatation, valvulitis or papillary muscle dysfunction. In addition to these symptoms and findings, there may occur several abnormalities, such as arrhythmia, heart block and supraventricular tachycardia caused by myocarditis of the conductive system [6].
Renal involvement was detected in 20% of patients with RP. It manifests itself predominantly in the form of segmental proliferative necrotizing glomerulonephritis. Immunofluorescence and electron microscopy examination showed a small amount of sediments of IgG and IgM immunoglobulins and of C3 component of the granular complement deposited in the subendothelial and mesangial areas. The course of the disease in patients with renal involvement is usually quite severe and may be associated with manifestations of extra-renal vasculitis, with an unfavourable prognosis [7].
Ocular involvement is another severe disorder in RP, particularly in the form of the eyeball inflammation, most often episcleritis, scleritis and corneal thinning that may cause perforations associated with other complications, leading ultimately to the loss of vision. Other ocular manifestations in RP that may also cause loss of vision include uveitis, retinal vasculitis, optic neuritis or eyeball protrusion as a result of vasculitis of the connective tissue behind the eyeball. In addition, this disease may be associated with paralysis of eye muscles, inflammation of the orbit and papilla swelling [6,8,9].
Skin symptoms in RP include purpura, urticaria and angioedema, less frequently livedo reticularis, migratory superficial thrombophlebitis, erythema nodosum, erythema multiforme and paniculitis [6].
Neurological complications in RP include cranial neuropathy, headache, encephalopathy, hemiplegia and ataxia, sometimes also transverse myelitis, mononeuritis multiplex and temporal non-granulomatous vasculitis.
In up to 22% of patients, RP is accompanied with quite a high fever. Bellamy and Dewar [10] described a case of a 25-year-old woman who presented with chondritis at 14 weeks of pregnancy. The patient was receiving high doses of glucocorticoids. Pregnancy and delivery of the child were physiological. The child developed normally after the birth and the disease did not re-activate in the postpartum period. Later the patient gave birth to another healthy child, again without re-activation of the disease.
Relapsing polychondritis in the elderly
Sallam, et al. [11] described an unusual manifestation of relapsing polychondritis presenting initially with isolated ocular signs, mimicking infective keratitis. It was a case report of a 75-year-old man who presented with marked left ocular irritation and photophobia. Ophthalmological examination disclosed corneal intrastromal infiltrate and hypopyon which failed to respond to intensive antimicrobial drops. Later the patient developed bilateral auricular chondritis and relapsing polychondritis was diagnosed. Treatment with topical and oral corticosteroids resulted in marked improvement of the corneal infiltrate and resolution of the auricular inflammation. The authors highlighted the importance of considering connective tissue inflammatory conditions in any stromal keratitis unresponsive to antimicrobial treatment, in the context of a developing relapsing polychondritis or another connective tissue inflammatory condition.
In 2010, Starr, et al. [12] presented a case report of a 70-year-old man with an unusual clinical manifestation of relapsing polychondritis in the form of alopecia areata.
Erten-Lyons, et al. [13] detected in a 50-year-old lawyer with the diagnosis of relapsing polychondritis subacute dementia associated with the disease. Another patient, a 68-year-old man, with the diagnosis of relapsing polychondritis presented with myalgia, headache, fever and bilateral swelling of auricles. During eight months the patient's condition was gradually aggravating, with typical weight loss, conjunctiva involvement and loss of cognitive functions. The patient was unable to perform activities of daily living without assistance and had speech difficulties lasting for several hours. Psychological examination revealed impaired verbal and visual memory indicating an early stage of dementia.
Incidence of relapsing polychondritis in association with other diseases
Relapsing polychondritis may occur simultaneously with ulcerative colitis, Behçet's syndrome, Wegener's granulomatosis, Sweet's syndrome, systemic lupus erythematosus and other inflammatory diseases of the connective tissue (rheumatoid arthritis, Sjogren's syndrome, systemic scleroderma, psoriatic arthritis, polyarteritis nodosa). The "Magic syndrome" (Mouth and Genital ulcers with Inflamed Cartilage syndrome) is a combination of the Behçet's disease and relapsing polychondritis [14] but occurs also in association with tumorous diseases, such as chronic lymphocytic leukemia [15].
Duda and Botka [16] described in a 64-year-old female patient a rare association of RP with Sjögren's syndrome, tubulointerstitial nephritis and autoimmune thyropathy. During one year tubulointerstitial nephritis was aggravated in the form of renal failure with hypokalemia and hyperuricemia. Later it was followed by a stroke and development of pancypenia even after elimination of cyclophosphamide from the treatment. After one year and 7 months the patient died of catheter related sepsis and bronchopneumonia.
The presented case reports indicate that a severe relapsing polychondritis may occur even in an older age category.
Laboratory findings
The common feature of laboratory parameters in RP is increase in the acute inflammatory phase reactants and the presence of anemia and thrombocytosis, sometimes also a mild leukocytosis. Serological tests have shown that the serum in 50% of patients was positive for antibodies against type II collagen. In most cases the finding includes circulating immune complexes and antibodies against intracellular antigens (approximately in 20% of cases). A finding of circulating anticoagulants was also described, which explains the clinical finding of severe thrombosis.
Diagnosis: RP should be considered as confirmed if the following clinical criteria are met:
1. Recurrent chondritis of both auricles;
2. Non-erosive polyarthritis;
3. Chondroitin of nasal cartilages;
4. Inflammation of ocular structures (including conjunctivitis, keratitis, scleritis, episcleritis and uveitis);
5. Involvement of laryngeal or tracheal cartilage;
6. Cochlear or vestibular damage.
The above mentioned criteria were published by McAdam, et al. [17], and later slightly modified by Damiani and Levine [18]. They include three or more of these criteria, however, the diagnosis requires at least one clinical criterion and a histological finding of chondritis in separate anatomical locations with response to the treatment. Based on analysis of 112 cases of the disease, the diagnostic criteria were modified in 1986 in Minnesota as follows:
RP has to be suspected when the inflammatory bouts involve at least two of the typical sites-auricular, nasal, laryngo-tracheal (or) one of the typical sites and two other - ocular, statoacoustic disturbances (hearing loss and/or vertigo) and arthritis.
Differential diagnosis
Although, the course of RP is quite typical, under certain conditions it may make the diagnosis difficult. It has to be taken into account that the auricle is highly sensitive to trauma, chemical agents, and frostbite. Similarly, trachea is highly sensitive to protracted endotracheal intubation. Similar symptoms as in perichondritis may be found in acute streptococcal infection, mycotic infection, syphilis and leprosy, which may be a cause of erroneous RP diagnosis. Therefore biopsy should be performed in order to establish a correct diagnosis. Nasal cartilage may be affected also by granulomatous processes (Wegener's granulomatosis, lymphomatoid granulomatosis, and lethal midline granuloma). Involvement of eyes may be manifested as necrotizing scleritis or keratitis also in inflammatory rheumatic diseases, such as RA, Wegener's granulomatosis, polyarteritis nodosa, Behçet's disease or Cogan's syndrome. Differential diagnosis must exclude pulmonary or renal vasculitis affecting CNS or other organs. The aortic root may be involved in case of Ehlers-Danlos syndrome, Marfan syndrome, idiopathic cystic medial necrosis associated with ankylosing spondylitis.
Therapy and prognosis
RP therapy depends on individual forms of the disease. Milder forms affecting the auricle or arthritis are treated with non-steroidal anti-inflammatory drugs and low doses of prednisone. Severe forms of the disease, such as laryngo-tracheal or ocular involvement, severe involvement of the ear and nasal cartilage, systemic vasculitis, aortitis or glomerulonephritis, require administration of prednisone at the dose of 1 mg/kg of body mass daily. In certain cases the prednisone doses may be successfully reduced and maintain reliable remission, while sometimes reduction of doses of glucocorticoids results in exacerbation of the disease [10]. In these cases a combined immunosuppressive treatment (cyclophosphamide, azathioprine, chlorambucil, and cyclosporine) should be attempted. Van der Lube, et al. [19] used anti-CD4 monoclonal antibody in the therapy. Recently, autologous transplantation of stem cells has been introduced in the RP treatment.
Within the basic RP treatment, Handler [20] used in a 68-year-old woman with confirmed RP diagnosis (with aurical involvement) 100 mg of leflunomide daily for three days, followed by 20 mg daily. Within two weeks the auricular inflammation resolved and the treatment reliably maintained remission for the period of three years [20,21]. In the resistant form, the inflammatory process could not be managed by glucocorticoids, methotrexate, azathioprine, antimalarials. Auricular and nasal cartilage was effectively treated with leflunomide at a dose of 20 mg and then 30 mg daily, however, the treatment had to be discontinued due to a febrile hematologic adverse response.
Recently, the resistant forms of RP have started to be treated with biological therapy by TNF inhibitors (infliximab, adalimumab, etanercept), anti-IL-6 receptor inhibitor (tocilizumab), Il-1 receptor inhibitor (anakinra) and rituximab (antibody against B-lymphocytes).
In 2002, Ehresmann [22] used infliximab in the treatment of a 35-year-old man with a 10-year history of RP. Due to resistance resulting from high doses of glucocorticoids in combination with MTX, the patient received infliximab at a dose of 5 mg/kg, in the form of infusion at week 0, 2, 6 and then every 8 weeks. MTX and glucocorticoids were gradually eliminated from the treatment. The therapy appeared to be safe and well tolerated. In 2010, Soares de Barros, et al. [23] treated a 42-year-old woman with RP, who developed resistance to therapy by glucocorticoids in combination with MTX and later azathioprine, with addition of infliximab at a dose of 3 mg/kg (dosage10,14,42). Her condition improved as early as after the second infusion. After the third infusion, arthritis subsided and the basal treatment could be radically reduced to 10 mg of prednisone and 7.5 mg of MTX weekly.
Carter [24] used etanercept in a 46-year-old female patient with a resistant form of RP, at a dose of 25 mg twice a week, in combination with glucocorticoids and MTX (20 mg daily). This therapy allowed gradual elimination of prednisone from the treatment (starting from the dose of 30 mg daily) and administration only of MTX at a dose of 15 mg daily. With combination of MTX and etanercept the disease remained clinically in remission. And finally, Seymour, et al. [25] successfully applied adalimumab at a dose of 40 mg every two weeks due to the disease relapse after previous treatment with infliximab for the period of ten months. Remission was maintained for four years of replacement of infliximab by adalimumab. This was the first case report of RP associated with aortitis where adalimumab was successfully used in the treatment.
Similar problems as in developed resistance of biological therapy to infliximab in RP, were addressed in resistance to classical immunosuppressive treatment (cyclosporine, azathioprine, cyclophosphamide and various doses of glucocorticoids). Infliximab proved to be inefficient after three infusions, and therefore anakinra was used the effect of which was immediate. It has been proved that anakinra may be an alternative drug in patients with a resistant form of RP requiring high doses of glucocorticoids to suppress the disease [26]. Kawai, et al. [27] pointed out the possibility of administration of tocilizumab in case of development of a resistant form of RP or exacerbation of the disease due to attempts at reduction of prednisone dosage.
However, there may occur also acute conditions during RP, such as acute airway obstruction. Such cases require aggressive treatment with pulse methylprednisolone at a dose of 1 g [28]. The patient must be followed up by a rheumatologist as well as by an otolaryngologist (indirect laryngoscopy, CT scanning of trachea, urgent tracheostomy for symptomatic subglottic stenosis). The disease may have fatal consequences in case of airway collapse of the bronchial stroma. Heart valve replacements and aortic grafts have also been used in the treatment.
Despite the above mentioned therapeutic possibilities, RP prognosis remains to be severe, especially in the forms affecting the laryngeal cartilages and individual organs (heart, respiratory system, and eye). Therefore rheumatologists should pay increased attention to this rare disease.
References
-
Jaksch-Wartenhorst R (1923) Polychondropathia. Wiener Archiv für Inn Med 6: 93-94.
-
Ebringer R, Rook G, Swana GT, Bottazzo GF, Doniach D (1981) Autoantibodies to cartilage and type II collagen in relapsing polychondritis and other rheumatic diseases. Ann Rheum Dis 40: 473-479.
-
Arundell FW, Haserick JR (1960) Familial chronic atrophic polychondritis. Arch Dermatol 82: 353-365.
-
Tomík F, Hajzok O, Rovenský J (1977) Recurrent polychondritis. Cesk Patol 13: 91-94.
-
Neilly JB, Winter JH, Stevenson RD (1985) Progressive tracheobronchial polychondritis: need for early diagnosis. Thorax 40: 78-79.
-
Longo L, Greco A, Rea A, Lo Vasco VR, De Virgilio A, et al. (2016) Relapsing polychondritis: A clinical update. Autoimmun Rev 15: 539-543.
-
Lee JE, Lee EK (2012) A case of membranous nephropathy associated with relapsing polychondritis. Kidney Res Clin Pract 31: 253-256.
-
Bernauer W, Pleisch B, Brunner M (2014) Five-year outcome in immune-mediated scleritis. Graefes Arch Clin Exp Ophthalmol 252: 1477-1481.
-
Mohsenin A, Huang JJ (2012) Ocular manifestations of systemic inflammatory diseases. Conn Med 76: 533-544.
-
Bellamy N, Dewar CL (1990) Relapsing polychondritis in pregnancy. J Rheumatol 17: 1525-1526.
-
Sallam A, Islam T, Parmar DN (2010) Keratouveitis as a first presentation of relapsing polychondritis. Case Rep Med.
-
Starr JC, Taneja N, Brasher GW (2010) Relapsing polychondritis following alopecia areata. Case Rep Med
-
Erten-Lyons D, Oken B, Woltjer R, Quinnn J (2009) Relapsing polychondritis: an uncommon cause of dementia. BMJ Case Rep.
-
Bochtler T, Hensel M, Lorenz HM, Ho AD, Mahlknecht U (2005) Chronic lymphocytic leukaemia and concomitant relapsing polychondritis: a report on one treatment for the combined manifestation of two diseases. Rheumatology (Oxford) 44: 1199.
-
Kim MK, Park KS, Min JK, Cho CS, Kim HY (2005) A case of polychondritis in a patient with Behçet's disease. Korean J Intern Med 20: 339-342.
-
Duda J, Botka M (2001) Unusual case of relapsing polychondritis. Rheumatologia 15: 136.
-
McAdam L, Lawrence P, O'Hanlan MA, Bluestone R, Pearson C (1976) Relapsing polychondritis: prospective study of 23 patients and a review of the literature. Medicine (Baltimore) 55: 193-215
-
Damiani JM, Levine HL (1979) Relapsing polychondritis--report of ten cases. Laryngoscope 89: 929-946.
-
van der Lubbe PA, Miltenburg AM, Breedveld FC (1991) Anti-CD4 monoclonal antibody for relapsing polychondritis. Lancet 337: 1349.
-
Handler RP (2006) Leflunomide for relapsing polychondritis: successful longterm teatment. J Rheumatol 33: 1916.
-
Koenig AS, Abruzzo JL (2002) Leflunomide induced fevers, thrombocytosis, and leukocytosis in a patient with relapsing polychondritis. J Rheumatol 29: 192-194.
-
Ehresmann GR (2002) Infliximab in the treatment of polychondritis. ACR Poster Session A, Pathogenesis and treatment of rare rheumatic diseases II.
-
de Barros AP, Nakamura NA, Santana Tde F, Motta JQ, Bianchi WA (2010) Infliximab in relapsing polychondritis. Rev Bras Reumatol 50: 211-116.
-
Carter JD (2005) Treatment of relapsing polychondritis with a TNF antagonist. J Rheumatol 32: 1413.
-
Seymour MW, Home DM, Wiliams RO, Allard SA (2007) Prolonged response to anti-tumour necrosis factor treatment with adalimumab (Humira) in relapsing polychondritis complicated by aortitis. Rheumatology 46: 1738-1739.
-
Vounotrypidis P, Sakellariou GT, Zisopoulos D, Berberidis C (2006) Refractory relapsing polychondritis: rapid and sustained response in the treatment with an IL-L receptor antagonist (anakinra). Rheumatology 45: 491-492.
-
Kawai M, Hagihara K, Hirano T, Shima Y, Kuwahara Y, et al. (2009) Sustained response to tocilizumab, anti-interleukine-6 receptor antibody, in two patients with refractory relapsing polychondritis. Rheumatology 48: 318-319.
-
Lipnick RL, Fink ChW (1991) Acute airway obstruction in relapsing polychondritis: Treatment with pulse methylprednisolone. J Rheumatol 18: 98-99.






