Journal of Rheumatic Diseases and Treatment
Pathogenesis of Bone Erosions in Rheumatoid Arthritis: Not Only Inflammation
Maurizio Rossini*, Angelo Fassio, Luca Idolazzi, Ombretta Viapiana, Elena Fracassi, Giovanni Adami, Maria Rosaria Povino, Maria Vitiello and Davide Gatti
Department of Medicine, Rheumatology Unit, University of Verona, Italy
*Corresponding author: Maurizio Rossini, Department of Medicine, Rheumatology Unit, Policlinico Borgo Roma, Piazzale Scuro, 10, 37134, Verona, Italy, Tel: +390458126770, Fax: +39O458126881, E-mail: maurizio.rossini@univr.it
J Rheum Dis Treat, JRDT-1-012, (Volume 1, Issue 2), Review Article; ISSN: 2469-5726
Received: March 31, 2015 | Accepted: May 19, 2015 | Published: May 22, 2015
Citation: Rossini M, Fassio A, Idolazzi L, Viapiana O, Fracassi E, et al. (2015) Pathogenesis of Bone Erosions in Rheumatoid Arthritis: Not Only Inflammation. J Rheum Dis Treat 1:012. 10.23937/2469-5726/1510012
Copyright: © 2015 Rossini M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
It is a matter of fact that the inflammatory pathogenesis does not explain all the skeletal manifestations of Rheumatoid Arthritis (RA), and the development of bone involvement is sometimes unconnected from the clinical scores of inflammation. On the basis of recent studies, we would like to make a point of the new available evidence about the metabolic pathogenic component of bone erosions. We assume that in this process an additional role could be played by metabolic factors, such as the parathyroid hormone (PTH), DKK1 (the inhibitor of the Wnt/β catenin pathway) and cortical bone mineral density. The result is a new pathogenic hypothesis for bone erosion in RA, which supplements the inflammatory one: the reduction of osteoblasts activity and the increase of the osteoclasts one, involved in the pathogenesis of bone erosions and osteoporosis, are not only the consequence of the action of inflammatory cytokines, but also of increased levels of DKK1 and PTH. On the other hand, osteoporosis, in particular at the cortical sites, facilitates the appearance of erosions.
Failing to assess and correct these metabolic alterations may explain an insufficient response in terms of prevention or healing of bone erosions to the disease modifying antirheumatic drugs.
Introduction
It has been suspected for so long that the three typical manifestations of bone involvement in rheumatoid arthritis (RA) (focal bone erosions, juxta-articular osteoporosis and systemic osteoporosis) could be the consequence of a common pathological mechanism [1] of both inflammatory and metabolic nature.
It is a matter of fact that the inflammatory pathogenesis does not explain all the skeletal manifestations of the rheumatic disease and the development of bone involvement is sometimes unconnected from the clinical scores of inflammation [2]. On the basis of recent studies in this review we would like to make a point of the new available evidence about the metabolic pathogenic component of bone erosions.
The Patogenesis of Bone Erosions: Not Only Inflammation
It is well known that at 5 years from the beginning of RA, 30-50% of patients has evidence of focal bone erosions [3]. This phenomenon is the result of many complex interactions from the cells and the cytokines/chemokines system at the synovial and surrounding tissues level, which culminates with bone destruction. In addition to the articular crumbling, RA is also associated with a generalized bone loss with a higher prevalence of osteoporosis: RA patients have double the normal risk of undergoing a femoral fracture [4] and they are 4 times more likely to have vertebral fractures [5,6]. Both erosions and systemic osteoporosis are related to the unbalance between osteoblasts and osteoclasts activity [7,8]. Some of the cytokines involved in RA physiopathology, as the tumor necrosis factor alpha (TNF-α) and RANKL, are also involved in the pathogenesis of both focal and systemic bone lesions [9,10]. The balance between the osteoblasts and osteoclasts activity in course of RA is conditioned not only from distinctive inflammatory factors (TNF-α, IL1, IL6, IL17, IFN-gamma...), but also from metabolic factors (IGF1, estradiol, parathyroid hormone, 1,25(OH)2D, leptin, ...) related to the disease per se or influenced by the use of some drugs as glucocorticoids.
The erosiveness of RA is typically very precocious and it is at the maximum level in the first years of the disease; the rapid establishing of the structural damage in RA is determined by a close interaction between the synovial joint membrane, cartilage and subchondral bone. The fundamental role is played by osteoclasts [8], both from the synovial and the medullary side [11]. The activation and replication of osteoclasts would be primarily linked to inflammation: a significant correlation between the inflammation score and the number of osteoclasts was shown [1].
During intense phlogosis the RANK-RANKL system is activated by inflammatory citokines (TNF-α, IL-1, IL-6, IL-17, PGE2) both at the medullary and at the synovial level and this generates the proliferation of osteoclasts [12,13]. Recently, it has been also described in RA patients an improved differentiation of the mononuclear cells of peripheral blood into osteoclasts, possibly due to an increase of osteoclasts blood precursors and a reduced apoptotic potential of mature osteoclasts [14]. An additional role could be played by the chronic increase of a metabolic factor such as the parathyroid hormone (PTH) which, along with 1,25(OH)2D, is a known stimulator of the production of RANLK and of osteoclasts activity [15,16].
Lately, it has also been observed that, despite osteoclasts are the main actors in the pathogenesis of bone erosions in RA, osteoblasts and their regulatory cytokines (especially dickkopf Wnt signaling pathway inhibitor 1, DKK1), whether directly or indirectly, participate too. In RA patients it was shown that an increase in serum DKK1 levels correlated positively with inflammation markers and with the Sharp scores [17]: considering the inhibitory effect of DKK1 on the Wnt system, and therefore on osteoblasts, this could represent a favorable condition for the erosive or the bone mineral density reducing the evolution of the disease.
DKK1 concentrations are not only influenced by inflammatory factors but also by mineral metabolic ones: for instance the long term effects of PTH on DKK1 [18] could justify an undesired suppression of osteoblasts activity in conditions of chronic hyperparathyroidism.
The Current Predictive Value of Bone Erosions is Low
Being able to predict a probably irreversible radiological damage or its progression is paramount to operate an early intervention and to evaluate the opportunity of a more or less aggressive treatment. The identification of specific risk factors could also enable the discovery of possible pathogenic components; besides, a modest predictive value of the already known risk factors could outline the opportunity to look for newer ones. A review of the past experiences has led to the proposal of predictive models for persistently erosive arthritis [19-22]. The main factors considered were: durations of symptoms (from 6 weeks and 6 months), morning stiffness ≥ 1 hour, joint count ("swollen" and "tender" joints) with arthritis in ≥ 3 joints, a positive "squeeze sign" of metatarsophalangeal and metacarpophalangeal joints, the positivity of rheumatoid factor, the positivity of anti-citrullinated antibodies, the presence of erosions at the hands and/or feet X-rays. In most of the studies conducted in early RA, a relevant factor for the prognosis is the finding of inflammation at the physical or instrumental examination (the number of swollen joints or typical sonographic or MRI abnormalities). Many studies have also taken into account the use of laboratory inflammation markers (such as ESR and CRP) as prognostic indicators in an early phase of the disease, since there is evidence of a correlation between the intensity and persistence of the inflammation and the progression of bone damage [23,24]. All this support the relevant pathogenic role played by inflammation in respect of bone erosions, focal or systemic. As a further confirmation of this it was recently observed that the repair of bone erosion can almost exclusively happen in non-swollen joints [25]. However, CRP could be a not so good predictive marker since this parameter is not infrequently normal in the early phases of the disease and some studies have also questioned the predictive ability of the determination of ESR and CRP in the early phase towards the development of radiological damage at one or two years.
It is also known that the polymyalgia-like onset RA, typical of the elderly, is characterized by high ESR and CRP values at the onset but a little tendency to develop short term erosions. Finally, there can even be radiological progression of the bone damage in absence of phlogosis signs, at least as detectable at physical examination [2]. Therefore inflammation does not explain everything about the bone damage in RA, or at least not every time.
On the other hand even the inclusion of the best prognostic known risk factors (RF and/or anti-CCP positivity, previous bone erosions), as well as inflammatory markers as CRP, to identify or exclude a significant risk of radiological progression, leads to 38% of false positives and 9% of false negatives [26].
Recently it was reported that even including in addition to the inflammatory markers 10 clinical variables (anti-CCP antibodies, RF, BMI, age, duration of symptoms, involvement of lower extremities, HLA, number of swollen joints, sex and anti-vimentin antibodies), only 32% of the bone erosions risk variance can be explained, thus confirming the modest predictive value in terms of erosive risk estimation through the multivariate model based on the actual known factors [27].
There is evidence that many other factors, besides inflammation, are acting and could interfere both in a pathogenic and in a protective extent concerning the bone involvement in RA and many of these are not well clarified yet. Note actually how, among the predictive factors of bone erosion, it's not considered any variable related to the bone or mineral metabolism.
Metabolic Factors Predictive For Bone Erosion
Markers of bone turnover, bone mineral density (BMD), PTH and DKK1 might represent new predictive biomarkers of bone erosions
Markers of bone turnover: Some parameters of the bone or cartilage metabolism have been investigated as possible markers or prognostic indicators of bone erosions [23,24,28-31]: they are the serum, urinary or synovial concentrations of many proteins connected to the bone or cartilage degradation (i.e. fragments of type I or II collagen, oligomeric proteins of the cartilage matrix) or regulators of osteoclasts (RANKL and osteoprotegerin) or osteoblasts activity (DKK1). For instance, it was observed that the urinary pirinolines and desoxypiridinolines excretion, expression of an increase in osteoclasts activity, relates with the scores of disease activity [29,30], confirming the role of the pro-inflammatory cytokines in the activation of bone reabsorption. In another study it was found that the erosive evolution in RA would occur in patients presenting higher levels of serum C-terminal telopeptide of type I collagen (CTX), a marker of osteoclastic resorption [31]. The clinical relevance of these limited observations is anyhow still uncertain.
Bone mineral density: Another possible candidate among the predictors of erosions could be bone mass or bone mineral density (Bone Mineral Density, BMD. The connection between RA and the reduction of BMD that is osteoporosis has gone defining over the years, although it has always been seen as the effect of RA or its pharmacological treatment on BMD, and not as the role of BMD towards the risk of bone erosions.
Traditionally in RA we speak of juxta-articular osteoporosis, generalized osteoporosis and, among the latter, drug-induced osteoporosis, corticosteroids in particular. The prevalence of osteoporosis in women with RA ranges from 30 to 50%, depending on the sites evaluated through bone densitometry [32]. If we also consider less severe conditions, such as osteopenia, the prevalence rises and touches 80%.
Bone mineral loss in RA appears to depend not only by the use of corticosteroids, although most of the available studies have been strongly conditioned by the use of corticosteroids: there are only few studies conducted in subjects who did not receive similar treatments, and either way always in less active diseases. On the other hand it should be considered that corticosteroid treatment in RA, through reducing the pathogenic component linked to phlogosis and especially if at lower dosages, could have neutral or even benefic effects, at last in the short-term, both regarding osteoporosis (notably the juxta-articular form [33]) and the erosion risk [34]. All this despite the well-known deleterious effects of corticosteroids towards bone (increase of bone resorption and inhibition of neoformation) and mineral metabolism (negativization of the calcium balance by reduction of the intestinal calcium absorption and increase of renal losses with consequent secondary hyperparathyroidism).
As already observed in 1994 at the vertebral level [35], recently as well it was reported that a high disease activity (evaluated through CRP or DAS28) represents an independent risk factor for hand BMD loss after 5 years [36].
In an Italian experience [32], besides confirming the deleterious effect of the corticosteroid treatment used in clinical practice and the classical risk factors for osteoporosis (menopause, age, BMI), an independent one was documented, represented by Health Assessment Questionnaire (HAQ). In premenopausal patients, in which we could exclude concomitant effects of an estrogenic deficiency, significantly reduced femur BMD values were reported [37], even after the correction for age, BMI and the cumulative dose of corticosteroids, thus confirming the disease per se as a risk factor for osteoporosis.
But what is the relationship between BMD and bone erosions? The correlation is clear and predictable if one evaluates the hand BMD, expression of a contingent juxta-articular osteoporosis. In some studies it was actually reported a statistically significant inverse correlation of the hand BMD and the prevalence of erosions [38]. There is available evidence in this sense also in terms of radiogrammetry: the cortical thickness and in particular the internal diameter evaluated at the level of the II metacarpal bone was seen to correlate significantly with the Sharp score for erosions [39]. The wrist BMD was noted to be inversely correlated with the prevalence of erosions too [40]. The latter were also seen to be correlated with axial or systemic osteoporosis. It is known that subjects who have an erosive disease present a higher incidence of osteoporosis, both at lumbar spine and femur [32,41,42]. Also Solomon et al. [43] investigated in a cross-sectional study the relationship between bone erosions and BMD in 163 postmenopausal women affected by RA [43]. It was observed a negative correlation between femur BMD and the erosion risk, but the significance of this correlation was lost at multivariate analysis when age, disease activity evaluated with DAS-CRP, BMI and the use of corticosteroids were included. The results of these studies suggest that the functional damage associated with erosions and therefore the disease severity could be responsible for the generalized osteoporosis. Most of these results derived from cross-sectional studies, with all the consequent limits implied.
In one of these studies, for instance, there was a significant correlation between the cortical hand BMD reduction evaluated through digitalized radiogrammometry and the development of erosions through time [44-46]. In another study this correlation was observed also with the reduction of systemic BMD and in particular at femur level [47]. Recently, a multicentric Italian study involving more than 20 rheumatology centers [48], besides confirming the role of the severity and the duration of the disease and the positivity of anti-CCP and Rheumatoid Factor as risk factors for bone erosions, documented that femur BMD is significantly lower in subjects with bone erosions than in those without them . Femur BMD remains significantly correlated to erosions even when the data were corrected for the main variables which could influence bone mass such as age, inflammatory markers (ESR, CRP), functional status (HAQ, ADL), BMI, blood 25(OH)D levels, corticosteroid or bisphosphonates therapy. These results are in contrast with the ones of Solomon et al. [43] but are justified by the large size of our series, 7 times higher.
The relationship between femoral BMD and erosions is better than the one observed between the latter and lumbar BMD [43,47-49]. This observation is, from a certain point of view, unexpected since the trabecular bone is more metabolically active and therefore a greater influence of proinflammatory cytokines would be expected on this site. On the other hand it is also known that with aging there is a loss of accuracy of the assessment of vertebral BMD due to concomitant osteoarthritis.
From another point of view, however, the greater correlation of erosions with the femur BMD, site of mainly cortical bone, is not surprising since these skeletal complications affect primarily this histological type of bone. The meaning of the direct correlation between femur BMD and the incidence of erosions appears to be scientifically and clinically relevant: if this observation, a result of a cross-sectional study, with all the consequent limits this implies, will be confirmed by longitudinal studies, it could mean that a high BMD, especially at the level of the cortical bone, has a protective role against the risk of bone erosion.
Actually, in a sub-analysis of the BeSt study the progression of the erosive score was faster in patients with lower BMD [47] and in an another recent study the radiographic erosion score after 3 years in patients with osteopenia/osteoporosis at baseline was double the patients with normal BMD [49]. Since there is also a positive regression between BMD and BMI, the correlation we found could justify the protective effect already described in several studies [43,50,51] of a high BMI against the risk of occurrence of bone erosions.
BMD and its variations, in addition to the traditional clinical mean of assessment of bone mass, could be proposed as indicator for severity and prognosis of erosive complications in RA, obviously to be placed side by side to the radiographic evaluation, ultrasound and magnetic resonance imaging.
Generalized osteoporosis and bone erosions are likely to share not only the same pathophysiological mechanisms, but also to influence each other. Therefore they require an even more combined therapeutic approach.
PTH: From physiopathology, the risk of osteoporosis associated to a chronic condition of hyperparathyroidism is well known, involving especially the cortical bone. Secondary hyperparathyroidism, probably, but not only, due to a vitamin D deficiency, is particularly common among RA patients [49]. It seems now quite relevant the recent observation that chronic higher levels of PTH may be directly associated, in RA, to a greater risk of erosions [48], perhaps due to a greater impairment of the cortical bone. This generates a new metabolic hypothesis of the pathogenesis of erosions which supplements the inflammatory one. The increased bone resorption and the contemporary reduction of bone formation, predisposing the erosions, could be attributed to the known chronic effects of PTH, stimulator of osteoclast activity and inhibitor of osteoblast activity. Moreover the involvement of PTH in the metabolic pathway of DKK1 could contribute to the pathogenesis of bone erosions [50].
DKK1: Recent studies documented the important role of DKK1, the physiological inhibitor of the Wnt system, in the regulation of periarticular bone remodeling [51,52]. The Wnt system is not only renowned for promoting bone neo-formation by osteoblasts but also for inhibiting bone resorption by osteoclasts [53-55].
Elevated levels of DKK1, under the stimulus of TNF-α, were found into the synovial fibroblasts and in the serum of RA patients [52,56] in whom they correlate with inflammatory markers and with the presence of bone erosions [17,50,57,58]. It has been demonstrated that some polymorphisms of genes regulating DKK1 expression affect the progression of bone erosions [59] and it also has been demonstrated that the DKK1 blockade could prevent bone erosions and the systemic loss of bone [60]. A chronic treatment with PTH induces an increase of serum DKK1 levels [18] and these positively correlate with the PTH levels in different clinical conditions [61-63], including RA [50].
The levels of DKK1 in RA also correlate inversely with BMD, in particular in the sites of mainly cortical bone as the femur [50].
Conclusions
Recent studies revealed an important role of some factors of bone metabolism in the pathogenesis of erosions. The result is a new pathogenic hypothesis for bone erosions in RA (Figure 1), which supplements the inflammatory one: the reduction of osteoblasts activity and the increase of the osteoclasts one, involved in the pathogenesis of bone erosions and osteoporosis, are not only the consequence of the action of inflammatory cytokines such as TNF-α, but also of increased levels of DKK1 resulting from inflammatory and also metabolic factors, including PTH. On the other hand osteoporosis, in particular the cortical involvement, facilitates the appearance of erosions.
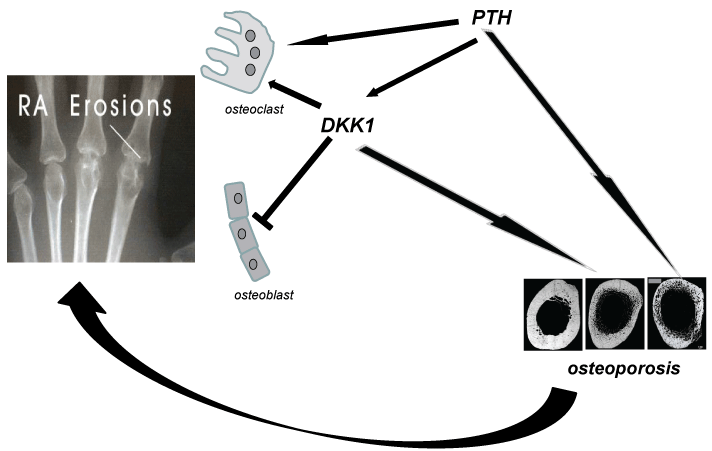
.
Figure 1: Metabolic pathogenesis of bone erosion. Chronic increase of PTH induces osteoclastogenesis, osteoporosis in cortical bone, and expression of DKK1. DKK1 itself leads to inhibition of osteoblast differentiation, and reduces focal and systemic bone formation. Osteoporosis of cortical bone increases the risk of bone erosions.
View Figure 1
Failing to assess and correct these metabolic alterations may explain an insufficient response in terms of prevention or healing of bone erosions to the drug treatment of RA.
Acknowledgments
The authors thank Sara Rossini who provided editorial assistance.
References
-
Sambrook PN (2000) The skeleton in rheumatoid arthritis: common mechanisms for bone erosion and osteoporosis? J Rheumatol 27: 2541-2542.
-
Molenaar ET, Voskuyl AE, Dinant HJ, Bezemer PD, Boers M, et al. (2004) Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 50: 36-42.
-
Lee DM, Weinblatt ME (2001) Rheumatoid arthritis. Lancet 358: 903-911.
-
Cooper C, Coupland C, Mitchell M (1995) Rheumatoid arthritis, corticosteroid therapy and hip fracture. Ann Rheum Dis 54: 49-52.
-
Orstavik RE, Haugeberg G, Uhlig T, Falch JA, Halse JI, et al. (2003) Vertebral deformities in 229 female patients with rheumatoid arthritis: associations with clinical variables and bone mineral density. Arthritis Rheum 49: 355-360.
-
Baskan BM, Sivas F, Alemdaroglu E, Duran S, Ozoran K. (2007) Association of bone mineral density and vertebral deformity in patients with rheumatoid arthritis. Rheumatol Int 27: 579-584.
-
Walsh NC, Gravallese EM (2004) Bone loss in inflammatory arthritis: mechanisms and treatment strategies. Curr Opin Rheumatol 16: 419-427.
-
Gravallese EM, Harada Y, Wang JT, Gorn AH, Thornhill TS, et al. (1998) Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am J Pathol 152: 943-951.
-
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, et al. (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304-309.
-
Redlich K, Görtz B, Hayer S, Zwerina J, Doerr N, et al. (2004) Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am J Pathol 164: 543-555.
-
Bugatti S, Caporali R, Manzo A, Vitolo B, Pitzalis C, et al. (2005) Involvement of subchondral bone marrow in rheumatoid arthritis: lymphoid neogenesis and in situ relationship to subchondral bone marrow osteoclast recruitment. Arthritis Rheum 52: 3448-3459.
-
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, et al. (2000) TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 106: 1481-1488.
-
Schett G, Stolina M, Bolon B, Middleton S, Adlam M, et al. (2005) Analysis of the kinetics of osteoclastogenesis in arthritic rats. Arthritis Rheum 52: 3192-3201.
-
Durand M, Boire G, Komarova SV, Dixon SJ, Sims SM, et al. (2011) The increased in vitro osteoclastogenesis in patients with rheumatoid arthritis is due to increased percentage of precursors and decreased apoptosis - the In Vitro Osteoclast Differentiation in Arthritis (IODA) study. Bone 48: 588-596.
-
Aubin JE, Bonnelye E (2000) Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Osteoporosis Int 11: 905-913.
-
Plum LA, DeLuca HF (2009) The functional metabolism and molecular biology of vitamin D action. Clin. Rev. Bone Miner. Metab 7: 20-41.
-
Wang SY, Liu YY, Ye H, Guo JP, Li R, et al. (2011) Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J Rheumatol 38: 821-827.
-
Gatti D, Viapiana O, Idolazzi L, Fracassi E, Rossini M, et al. (2011) The waning of teriparatide effect on bone formation markers in postmenopausal osteoporosis is associated with increasing serum levels of DKK1. J Clin Endocrinol Metab 96: 1555-1559.
-
van der Helm-van Mil AH1, Detert J, le Cessie S, Filer A, Bastian H, et al. (2008) Validation of a prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: moving toward individualized treatment decision-making. Arthritis Rheum 58: 2241-2247.
-
Kuriya B, Cheng CK, Chen HM, Bykerk VP (2009) Validation of a prediction rule for development of rheumatoid arthritis in patients with early undifferentiated arthritis. Ann Rheum Dis 68: 1482-1485.
-
Mjaavatten M, Van der Helm-van Mil A, Huizinga T (2009) Validation of a proposed prediction rule for rheumatoid arthritis in a cohort of 188 patients with undifferentiated arthritis. Ann Rheum Dis 67: 1482-1485.
-
Vastesaeger N, Xu S, Aletaha D, St Clair EW, Smolen JS (2009) A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford) 48: 1114-1121.
-
Lindqvist E, Eberhardt K, Bendtzen K, Heinegċrd D, Saxne T (2005) Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 64: 196-201.
-
Emery P, Gabay C, Kraan M, Gomez-Reino J (2007) Evidence-based review of biologic markers as indicators of disease progression and remission in rheumatoid arthritis. Rheumatol Int 27: 793-806.
-
Lukas C, van der Heijde D, Fatenajad S, Landewé R (2010) Repair of erosions occurs almost exclusively in damaged joints without swelling. Ann Rheum Dis 69: 851-855.
-
Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Ronday HK, Seys PE, et al. (2010) A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 69: 1333-1337.
-
de Rooy DP, van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH (2011) Predicting arthritis outcomes--what can be learned from the Leiden Early Arthritis Clinic? Rheumatology (Oxford) 50: 93-100.
-
van Tuyl LH, Voskuyl AE, Boers M, Geusens P, Landewé RB, et al. (2010) Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann Rheum Dis 69: 1623-1628.
-
Oelzner P, Müller A, Deschner F, Hüller M, Abendroth K, et al. (1998) Relationship between disease activity and serum levels of vitamin D metabolites and PTH in rheumatoid arthritis. Calcif Tissue Int 62: 193-198.
-
Oelzner P, Lehmann G, Eidner T, Franke S, Müller A, et al. (2006) Hypercalcemia in rheumatoid arthritis: relationship with disease activity and bone metabolism. Rheumatol Int 26: 908-915.
-
Garnero P, Landewe R, Boers M, Verhoeven A, Van Der Linden S, et al. (2002) Association of baseline levels of markers of bone and cartilage degradation with long-term progression of joint damage in patients with early rheumatoid arthritis: the COBRA study. Arthritis Rheum 46: 2847-2856.
-
Sinigaglia L, Nervetti A, Mela Q, Bianchi G, Del Puente A, et al. (2000) A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol 27: 2582-2589.
-
Haugeberg G, Strand A, Kvien TK, Kirwan JR (2005) Reduced loss of hand bone density with prednisolone in early rheumatoid arthritis: results from a randomized placebo-controlled trial. Arch Intern Med 165: 1293-1297.
-
Kirwan JR, Bijlsma JW, Boers M, Shea BJ (2007) Effects of glucocorticoids on radiological progression in rheumatoid arthritis. Cochrane Database Syst Rev: CD006356.
-
Gough AK, Lilley J, Eyre S, Holder RL, Emery P (1994) Generalised bone loss in patients with early rheumatoid arthritis. Lancet 344: 23-27.
-
Hoff M, Boyesen P, Haugeberg G, Vis M, Woolf AD, et al. (2010) High disease activity is a predictor of cortical hand bone loss in post-menopausal patients with established rheumatoid arthritis: a 5-year multicentre longitudinal study. Rheumatology (Oxford) 49: 1676-1682.
-
Benini C, Idolazzi L, Zambarda C, Mattarei A, Troplini S, et al. (2011) Bone mass and metabolism in pre-menopausal women with rheumatoid artritis. Ann Rheum Dis 70: 714.
-
Haugeberg G, Lodder MC, Lems WF, Uhlig T, Orstavik RE, et al. (2004) Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50-70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann Rheum Dis 63: 1331-1334.
-
Chan E, Pandith V, Towheed TE, Brouillard D, Zee B, et al. (1998) Comparison of the combined cortical thickness of the second metacarpal with Sharp's method for scoring hand microradiographs in rheumatoid arthritis. J Rheumatol 25: 1290-1294.
-
Sivas F, Barça N, Onder M, Ozoran K (2006) The relation between joint erosion and generalized osteoporosis and disease activity in patients with rheumatoid arthritis. Rheumatol Int 26: 896-899.
-
Lodder MC, Haugeberg G. Lems WF, Uhlig T, Orstavik RE, et al. (2003). Radiographic damage associated with low bone mineral density and vertebral deformities in rheumatoid arthritis: the Oslo-Truro-Amsterdam (OSTRA) Collaborative Study. Arthritis Rheum 49: 209-215.
-
Lodder MC, de Jong Z, Kostense PJ, Molenaar ET, Staal K, et al. (2004) Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann Rheum Dis 63: 1576-1580.
-
Solomon DH, Finkelstein JS, Shadick N, LeBoff MS, Winalski CS, et al. (2009) The relationship between focal erosions and generalized osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum 60: 1624-1631.
-
J Jensen T, Klarlund M, Hansen M, Jensen KE, Pĝdenphant J, et al. (2004) Bone loss in unclassified polyarthritis and early rheumatoid arthritis is better detected by digital x ray radiogrammetry than dual x ray absorptiometry: relationship with disease activity and radiographic outcome. Ann Rheum Dis 63: 15-22.
-
Stewart A, Mackenzie LM, Black AJ, Reid DM (2004) Predicting erosive disease in rheumatoid arthritis. A longitudinal study of changes in bone density using digital X-ray radiogrammetry: a pilot study. Rheumatology (Oxford) 43: 1561-1564.
-
Hoff M, Haugeberg G, Odegċrd S, Syversen S, Landewé R, et al. (2009) Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann Rheum Dis 68: 324-329.
-
Güler-Yüksel M, Allaart CF, Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Groenendael JH, et al. (2009) Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann Rheum Dis 68: 330-336.
-
Rossini M, Bagnato G, Frediani B, Iagnocco A, LA Montagna G, et al. (2011) Relationship of focal erosions, bone mineral density, and parathyroid hormone in rheumatoid arthritis. J Rheumatol 38: 997-1002.
-
Rossini M, Maddali Bongi S, La Montagna G, Minisola G, Malavolta N, et al. (2010) Vitamin D deficiency in rheumatoid arthritis: prevalence, determinants and associations with disease activity and disability. Arthritis Res Ther 12: R216.
-
Rossini M, Viapiana O, Adami S, Fracassi E, Idolazzi L, et al. (2015) In patients with rheumatoid arthritis, Dickkopf-1 serum levels are correlated with parathyroid hormone, bone erosions and bone mineral density. Clin Exp Rheumatol 33: 77-83.
-
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148: 2635-2643.
-
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, et al. (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13: 156-163.
-
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, et al. (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751-764.
-
Spencer GJ, Utting JC, Etheridge SL, Arnett TR, Genever PG (2006) Wnt signalling in osteoblasts regulates expression of the receptor activator of NFkappaB ligand and inhibits osteoclastogenesis in vitro. J Cell Sci 119: 1283-1296.
-
Walsh NC, Reinwald S, Manning CA, Condon KW, Iwata K, et al. (2009) Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res 24: 1572-1585.
-
Voorzanger-Rousselot N, Ben-Tabassi NC, Garnero P (2009) Opposite relationships between circulating Dkk-1 and cartilage breakdown in patients with rheumatoid arthritis and knee osteoarthritis. Ann Rheum Dis 68: 1513-1514.
-
Garnero P, Tabassi NC, Voorzanger-Rousselot N (2008) Circulating dickkopf-1 and radiological progression in patients with early rheumatoid arthritis treated with etanercept. J Rheumatol 35: 2313-2315.
-
Liu YY, Long L, Wang SY, Guo JP, Ye H, et al. (2010) Circulating Dickkopf-1 and osteoprotegerin in patients with early and longstanding rheumatoid arthritis. Chin Med J (Engl) 123: 1407-1412.
-
de Rooy DP, Yeremenko NG, Wilson AG, Knevel R, Lindqvist E, et al. (2013) Genetic studies on components of the Wnt signalling pathway and the severity of joint destruction in rheumatoid arthritis. Ann Rheum Dis 72: 769-775.
-
Heiland GR, Zwerina K, Baum W, Kireva T, Distler JH, et al. (2010) Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis 69: 2152-2159.
-
Rossini M, Gatti D, Adami S (2013) Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int 93: 121-132.
-
Viapiana O, Fracassi E, Troplini S, Idolazzi L, Rossini M, et al. (2013) Sclerostin and DKK1 in primary hyperparathyroidism. Calcif Tissue Int 92: 324-329.
-
Rossini M, Viapiana O, Zanotti R, Tripi G, Perbellini O, et al. (2015) Dickkopf-1 and sclerostin serum levels in patients with systemic mastocytosis. Calcif Tissue Int 96: 410-416.





